polymer → polimer
Polymer is a substance composed of molecules of high relative molecular mass (molecular weight), the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass (monomers). In most cases the number of monomers is quite large and is often not precisely known. A single molecule of a polymer is called a macromolecule. Polystyrene is light solid material obtained by polymerisation of styrene (vinyl benzene).
potassium → kalij
Potassium was discovered by Sir Humphry Davy (England) in 1807. The origin of the name comes from the Arabic word qali meaning alkali (the origin of the symbol K comes from the Latin word kalium). It is soft, waxy, silver-white metal. Fresh surface has silvery sheen. Quickly forms dull oxide coating on exposure to air. Reacts strongly with water. Reacts with water to give off flammable gas. Reacts violently with oxidants. Occurs only in compounds. Potassium is found in minerals like carnallite [(KMgCl3)·6H2O] and sylvite (KCL). Pure metal is produced by the reaction of hot potassium chloride and sodium vapours in a special retort. Used as potash in making glass and soap. Also as saltpetre, potassium nitrate (KNO3) to make explosives and to colour fireworks in mauve. Vital to function of nerve and muscle tissues.
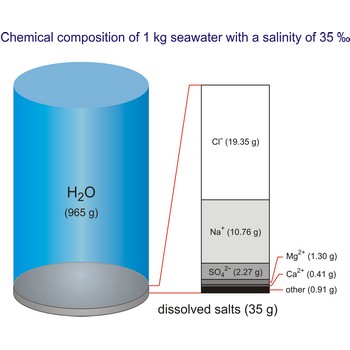
salt water → slana voda
Salt water is the water of the sea and the ocean. This water contains a relatively high percentage of dissolved salt (about 35 g of salt per 1 000 g of sea water.). About 90 % of that salt would be sodium chloride, or ordinary table salt.
The salinity of ocean water varies. It is affected by such factors as melting of ice, inflow of river water, evaporation, rain, etc.
ribonucleic acid → ribonukleinska kiselina
Ribonucleic acid is a complex organic compound in living cells that is concerned with protein synthesis. Plays an intermediary role in converting the information contained in DNA into proteins. RNA carries the genetic information from DNA to those parts of the cell where proteins are made. Some viruses store their genetic information as RNA not as DNA.
Ribonucleic acid is a similar molecule to DNA but with a slightly different structure.
The structural difference with DNA is that RNA contains a -OH group both at the 2' and 3' position of the ribose ring, whereas DNA (which stands, in fact, for deoxy-RNA) lacks such a hydroxy group at the 2' position of the ribose. The same bases can be attached to the ribose group in RNA as occur in DNA, with the exception that in RNA thymine does not occur, and is replaced by uracil, which has an H-group instead of a methyl group at the C-5 position of the pyrimidine. Unlike the double-stranded DNA molecule, RNA is a single-stranded molecule.
The three main functionally distinct varieties of RNA molecules are: (1) messenger RNA (mRNA) which is involved in the transmission of DNA information, (2) ribosomal RNa (rRNA) which makes up the physical machinery of the synthetic process, and (3) transfer RNA (tRNA) which also constitutes another functional part of the machinery of protein synthesis.
Schellbach’s burette → Schellbachova bireta
When colourless liquids are used, parallax mistake is avoided by use of Schellbach’s burette. On the inside wall opposite to graduation scale it has a melted in ribbon from milky glass in the middle of which a blue line is found. The level of liquid is now spotted very easily because of light breaking in the meniscus blue line now looks like a double spike.
standard electrode potential → standardni elektrodni potencijal
Standard electrode potential (E°) (standard reduction potentials) are defined by measuring the potential relative to a standard hydrogen electrode using 1 mol solution at 25 °C. The convention is to designate the cell so that the oxidised form is written first. For example,
The e.m.f. of this cell is -0.76 V and the standard electrode potential of the Zn2+|Zn half cell is -0.76 V.
sugar → šećer
Sugar is any of a group of water-soluble carbohydrates of relatively low molecular weight and typically having a sweet taste. The group comprises mainly monosaccharides (glucose, fructose, galactose), disaccharides (sucrose, lactose, maltose), and trisaccharides (raffinose). Many monosaccharides and disaccharides fairly commonly found in nature bear names reflecting the source from which they were first isolated. For example, glucose is also known as grape sugar, lactose as milk sugar, and maltose as malt sugar. In everyday usage, the name is often used to refer specifically to sucrose (table sugar, cane sugar, beet sugar).
Citing this page:
Generalic, Eni. "Relativna pogreška." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table