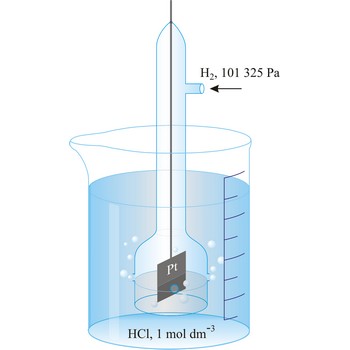
standard hydrogen electrode → standardna vodikova elektroda
Standard hydrogen electrode is a system in which hydrogen ion and gaseous hydrogen are present in their standard states. The convention is to designate the cell so that the standard hydrogen electrode is written first.
The electrode is used as a reference (of zero) for the values of other standard electrode potentials.
Heyrovsky-Ilkovic equation → Heyrovsky-Ilkovičeva jednadžba
The Heyrovsky-Ilkovic equation describes the entire current-potential curve (polarographic wave) of a reversible redox system in polarography
where R is the gas constant, T is the absolute temperature, F is the Faraday constant, n denotes the number of electrons taking part in the electrode reaction. E1/2 is a unique potential (for a given reaction and supporting electrolyte) termed the half-wave potential.
In order to obtain E1/2 from the above equation, we plot a graph of ln[(id-i)/i] against E. The intercept on the x-axis gives then an accurate value of E1/2. The slope of the obtained straight line is equal to nF/RT from which n is determined.
Citing this page:
Generalic, Eni. "Redoks." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table