termination → terminacija
Termination is the final step in a free radical mechanism that results in the stopping of the free radical reaction.
theoretical yield → teoretski prinos
Theoretical yield is the maximum quantity of a product that could be formed in a chemical reaction if all the limiting reactants reacted to form products (distinguished from actual yield).
theories of catalysis → teorije katalize
Theories of catalysis explain the influence of the catalysts upon the rate of a reaction by describing the detailed mechanism by which the catalyst is involved in the steps of the chemical reaction.
thermit welding → termitno zavarivanje
Thermit welding is a group of welding processes in which fusion is produced by heating with superheated liquid metal resulting from a chemical reaction between a metal oxide and aluminium.
Hesse’s law → Hessov zakon
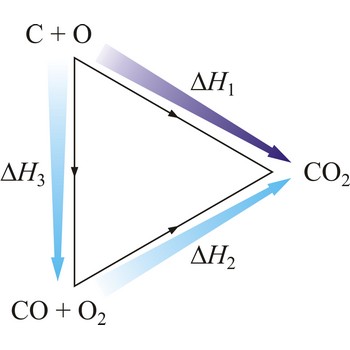
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
hydrogen → vodik
Hydrogen was discovered by Sir Henry Cavendish (England) in 1766. The origin of the name comes from the Greek words hydro and genes meaning water and generate. It is colourless, odourless gas, burns and forms explosive mixtures in air. Reacts violently with oxidants. Hydrogen is the most abundant element in the universe. Commercial quantities of hydrogen are produced by reacting superheated steam with methane or carbon. In lab work from reaction of metals with acid solutions or electrolysis. Most hydrogen is used in the production of ammonia and in metal refining. Also used as fuel in rockets. Its two heavier isotopes (deuterium and tritium) used respectively for nuclear fusion.
thermochemistry → termokemija
Termochemistry is the study of heat absorbed or released during chemical changes.
titrant → titrant
Titrant is the substance that quantitatively reacts with the analyte in a titration. The titrant is usually a standard solution added carefully to the analyte until the reaction is complete. The amount of analyte is calculated from the volume and concentration of titrant required for the complete reaction.
valence shell → valentna ljuska
Valence shell is the shell corresponding to the highest value of principal quantum number in the atom. The valence electrons in this shell are on average farther from the nucleus than other electrons. They are often directly involved in chemical reaction.
hydrolysis → hidroliza
Hydrolysis is a chemical reaction in which water reacts with another substance to form two or more new substances. This involves ionisation of the water molecule, as well as splitting of the compound hydrolysed, e.g.
Examples are conversion of starch to glucose by water in the presence of suitable catalysts and a reaction of the ions of a dissolved salt to form various products, such as acids, complex ions, etc.
Citing this page:
Generalic, Eni. "Reakcije adicije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table