Results 1–10 of 10 for raspadanje
decomposition → raspadanje
Decomposition occurs when chemical compounds are broken up into simple molecules, and even as far as their original elements. These processes are normally irreversible. An example of decomposition is when ammonium nitrate is heated. This produces nitrous oxide and water which are unable to recombine.
coal → ugljen
Coal is a black or brownish-black, combustible sedimentary rock, with 30 % (lignite) to 98 % (anthracite) carbon by weight, mixed with various amounts of water and small amounts of sulfur and nitrogen compounds. It is formed from plant matter that decayed in swamps and bogs that has been compressed and altered by geological processes over millions of years. Coal is primarily used as a fuel.
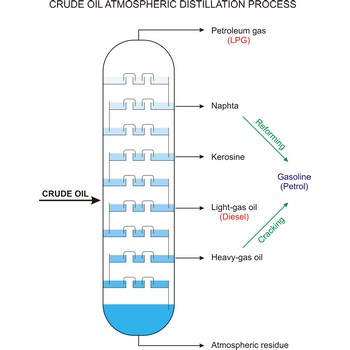
crude oil → sirova nafta
Crude oil (petroleum) is a fossil fuel formed from plant and animal remains many million of years ago. It is occasionally found in springs or pools but is usually drilled from wells beneath the earth’s surface. Crude oil is a mixture of hydrocarbons with small quantities of other chemicals such as sulphur, nitrogen and oxygen. Crude is the raw material which is refined into petrol, heating oil, jet fuel, propane, petrochemicals, and other products.
Curie → Curie
Maria Sklodowska-Curie (1867-1934) Polish-born French chemist who went to Paris in 1891. She married the physicist Pierre Curie (1859-1906) in 1985 and soon began work on seeking radioactive elements other than uranium in pitchblende (to account for its unexpectedly high radioactivity). By 1898 she had discovered radium and polonium although it took her years to purify them. In 1903 the Curies shared the Nobel Prize for physics with Henri Becquerel, who had discovered radioactivity.
radioactivity → radioaktivnost
Radioactivity is capability of a spontaneous decay of an atom. In this way a new atom type is formed and radioactive radiation is released. An atom can emit three types of radioactive radiation: positive α-radiation, negative β-radiation and electrically neutral γ-radiation. During radioactive decay one element never emits all types of radiation at the same time.
decay series → raspadni niz
Decay series is a series of decay in which radioactive element is decomposed in different elements until it produces one stable atom.
half-life → vrijeme poluraspada
For a simple radioactive decay process, half-life, t1/2, is defined as the time required for the activity of a given radioactive isotopes to decrease to half its value by that process.
The half-life is a characteristic property of each radioactive isotope and is independent of its amount or condition.
Citing this page:
Generalic, Eni. "Raspadanje." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table