radioactive indicator → radioaktivni indikator
By use of suitable radioactive isotopes biochemical processes can be observed in plants, animals and humans, by measuring radioactive radiation of radioactive indicator. Artificial radioactive isotopes have the same chemical properties as natural ones, which enable us to mark those natural isotopes with addition of artificial ones and in this way follow the path of those elements during a chemical reaction. One of the most important radioactive indicators is the radioactive carbon 14C.
radioactive series → radioaktivni niz
Radioactive series is a sequence of nuclides formed by successive radioactive decays until a stable decay product, the end product, is formed. A famous example of a radioactive series is the decay of uranium, which through a series of steps decays into stable lead.
radium → radij
Radium was discovered by Marie and Pierre Curie (France) in 1898. The origin of the name comes from the Latin word radius meaning ray. It is silvery-white radioactive metal. Reacts with oxygen and water. Highly radiotoxic. Carcinogen by inhalation, ingestion, or exposure. Radium is found in uranium ores at 1 part per 3 million parts uranium. Used in treating cancer because of the gamma rays it gives off.
visible radiation → vidljivo zračenje
Human eye can only see electromagnetic radiation of wavelengths form 400 nm to 760 nm. This narrow part of electromagnetic spectrum is called visible radiation. Visible (white) light is a mixture of light of all kind of colours, it can be separated, with the help of a glass prism, into its component colours - visible light spectrum, and each colour corresponds to a certain area of wavelengths:
| Colour | Wavelength / nm |
|---|---|
| purple | 400 - 450 |
| blue | 450 - 500 |
| green | 500 - 570 |
| yellow | 570 - 590 |
| orange | 590 - 620 |
| red | 620 - 760 |
absorbance → apsorbancija
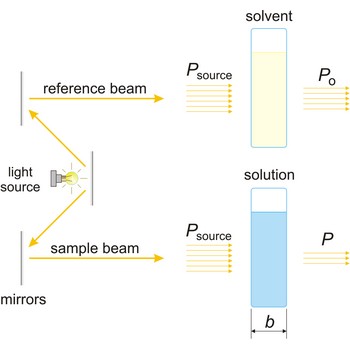
Absorbance (A) is a logarithm of the ratio of incident radiant power (Po) to transmitted radiant power (P) through a sample (excluding the effects on cell walls).
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
absorbed dose → apsorbirana doza
For any ionising radiation, absorbed dose (D) is the mean energy imparted to an element of irradiated matter divided by the mass of that element.
absorptance → apsorptancija
Absorptance (α) is the ratio of the radiant or luminous flux in a given spectral interval absorbed in a medium to that of the incident radiation. Also called absorption factor.
absorption coefficient → apsorpcijski koeficijent
Absorption coefficient (a) is the relative decrease in the intensity of a collimated beam of electromagnetic radiation, as a result of absorption by a medium, during traversal of an infinitesimal layer of the medium, divided by the length traversed.
acid salt → kisela sol
Acid salt is a compound formed by replacing hydrogen in an acid with a metal (or a radical that acts like a metal).
actinides → aktinoidi
Actinides (actinons or actinoids) are the fourteen elements from thorium to lawrencium inclusive, which follow actinium in the periodic table. The position of actinium is somewhat equivocal and, although not itself an actinide, it is often included with them for comparative purpose. The series includes the following elements: thorium (Th), protactinium (Pa), uranium (U), neptunium (Np), plutonium (Pu), amercium (Am), curium (Cm), berkelium (Bk), californium (Cf), einsteinium (Es), fermium (Fm), mendelevium (Md), nobelium (No) and lawrencium (Lr). Every known isotope of the actinide elements is radioactive. Traces of Pa, Np and Pu are consequently found, but only Th and U occur naturally to any useful extent.
Citing this page:
Generalic, Eni. "Radij." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table