infrared radiation → infracrveno zračenje
Infrared radiation is an electromagnetic radiation within the area from 1.0 μm to 300 μm, and is responsible for the transmission of radiant heat.
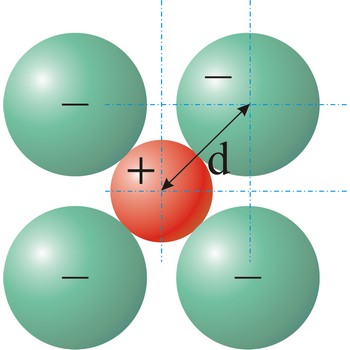
ionic radius → ionski radijus
Ionic radius is the radius of anions and cations in crystalline ionic compounds, as determined by consistently partitioning the center-to-center distance of ions in those compounds. In general, negative ions have larger ionic radii than positive ions.
radiant power → snaga radijacije
Radiant power is energy of radiation striking a unit area per unit time. The SI unit of radiant power is J m-2 s-1.
radiation → radijacija
The process of heat transfer which occurs through empty space and can also occur in matter, in the form of electro-magnetic (EM) waves, is called radiation or radiant heat. Whenever EM radiation is emitted and then absorbed, heat is transferred. This principle is used in microwave ovens, laser cutting, and RF hair removal.
radiation damage → radijacijsko oštećenje
Radiation damage is a general term for the alteration of properties of a material arising from exposure to ionising radiation (penetrating radiation), such as X-rays, γ-rays, neutrons, heavy-particle radiation, or fission fragments in the nuclear fuel material.
radioactivity → radioaktivnost
Radioactivity is capability of a spontaneous decay of an atom. In this way a new atom type is formed and radioactive radiation is released. An atom can emit three types of radioactive radiation: positive α-radiation, negative β-radiation and electrically neutral γ-radiation. During radioactive decay one element never emits all types of radiation at the same time.
radiography → radiografija
Radiography is nondestructive method of internal examination in which metal objects are exposed to a beam of X-ray or gamma radiation. Differences in thickness, density, or absorption caused by internal defects or inclusions are apparent in the shadow image produced on a fluorescent screen or photographic film placed behind the object.
van der Waals’s radius → van der Waalsov radijus
Van der Waals’s radius is one half the distances between two nonbonded atoms, when attractive and repulsive forces between the atoms are balanced.
Citing this page:
Generalic, Eni. "Radij." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table