proton → proton
Proton is a stable elementary particle of unit positive charge and spin 1/2. Protons and neutrons, which are collectively called nucleons, are the constituents of the nucleus.
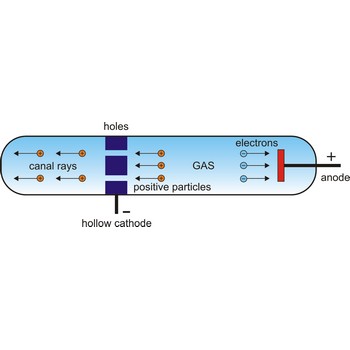
In 1886, German physicist Eugene Goldstein (1850-1930) discovered positive particles by using a modified Crookes tube with holes in the cathode in an evacuated tube. When cathode rays were given off in one direction toward the anode, other rays found their way through the holes in the cathode and sped off in the opposite direction. Since these other rays traveled in the direction opposite to the negatively charged cathode rays, it seemed that they must be composed of positively charged particles. Rutherford suggested that this fundamental positive particle be called the proton.
accelerator → akcelerator
Accelerator is a device (machine) used for acceleration of charged particles (protons, deuterons, α-particles). Particles are accelerated under the influence of an electric field and with the help of a magnetic field are kept inside a certain space. When the particles reach enough acceleration (that is sufficient energy), they are directed on a target we wish to bomb. Best known types cyclotron, synchrotron, betatron.
Accelerator is a substance that increases the rate of chemical reaction, i.e. a catalyst.
acid → kiselina
Acid is a type of compound that contains hydrogen and dissociates in water to produce positive hydrogen ions. The reaction for an acid HA is commonly written:
In fact, the hydrogen ion (the proton) is solvated, and the complete reaction is:
This definition of acids comes from the Arrhenius theory. Such acids tend to be corrosive substances with a sharp taste, which turn litmus red and produce colour changes with other indicators. They are referred to as protonic acids and are classified into strong acids, which are almost completely dissociated in water, (e.g. sulphuric acid and hydrochloric acid), and weak acids, which are only partially dissociated (e.g. acetic acid and hydrogen sulphide). The strength of an acid depends on the extent to which it dissociates, and is measured by its dissociation constant.
In the Lowry-Brønsted theory of acids and bases (1923), the definition was extended to one in which an acid is a proton donor (a Brønsted acid), and a base is a proton acceptor (a Brønsted base). An important feature of the Lowry-Brønsted concept is that when an acid gives up a proton, a conjugate base is formed that is capable of accepting a proton.
Similarly, every base produces its conjugate acid as a result of accepting a proton.
For example, acetate ion is the conjugate base of acetic acid, and ammonium ion is the conjugate acid of ammonia.
As the acid of a conjugate acid/base pair becomes weaker, its conjugate base becomes stronger and vice versa.
A further extension of the idea of acids and bases was made in the Lewis theory. In this, a G. N. Lewis acid is a compound or atom that can accept a pair of electrons and a Lewis base is one that can donate an electron pair. This definition encompasses "traditional" acid-base reactions, but it also includes reactions that do not involve ions, e.g.
in which NH3 is the base (donor) and BCl3 the acid (acceptor).
atomic number → atomski broj
Atomic number (Z) is a characteristic property of an element, equal to the number of protons in the nucleus. The atomic number and the element symbol are two alternate ways to label an element. In nuclide symbols, the atomic number is a leading subscript; for example, in 2He.
Bronsted-Lowry’s acid-base theory → Bronsted-Lowryeva teorija kiselina i lužina
Brønsted-Lowry’s acid-base theory: Acid is a substance which gives a proton (protondonor) and base is a substance which accepts a proton (protonacceptor).
alpha particle → alfa-čestica
Alpha particle is a helium nucleus emitted spontaneously from radioactive elements, both natural and manufactured. Its energy is in range 4-8 MeV and is dissipated in a very short path, i.e. a few centimetres of air or less than 0.005 mm of aluminium. As helium nucleus consists of two protons and two neutrons bound together as a stable entity the loss of an alpha particle involves a decrease in nucleon number of 4 and decrease of 2 in the atomic number, e.g.
A stream of alpha particles is known as an alpha ray or alpha-radiation.
atom → atom
Atom is an atom is the smallest particle of an element that retains the chemical properties of the element. Rutherford-Bohr’s model represents the atom as a positively charged core of a size around 10-14 m composed of protons (positive particles) and neutrons (neutral particles) around which negatively charged electrons circle. The number of protons and electrons are equal, so the atom is an electrically a neutral particle. Diameter of the atom is about 10-10 m.
base → baza
Historically, base is a substance that yields an OH - ion when it dissociates in solution, resulting in a pH>7. In the Brønsted definition, a base is a substance capable of accepting a proton in any type of reaction. The more general definition, due to G.N. Lewis, classifies any chemical species capable of donating an electron pair as a base. Typically, bases are metal oxides, hydroxides, or compounds (such as ammonia) that give hydroxide ions in aqueous solution.
beta particle → beta-čestica
Beta particle is a charged particle emitted from a radioactive atomic nucleus either natural or manufactured. The energies of beta particles range from 0 MeV to 4 MeV. They carry a single charge; if this is negative, the particle is identical with an electron; if positive, it is a positron.
An unstable atomic nucleus changes into a nucleus of the same mass number but different proton number with the emission of an electron and an antineutrino (or a positron and a neutrino)
conjugated acid → konjugirana kiselina
Conjugated acid is a particle that develops after a base receives a proton.
Citing this page:
Generalic, Eni. "Proton." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table