flash point → temperatura zapaljenja
Flash point is the lowest temperature at which a liquid or volatile solid gives off vapour sufficient to form an ignitable mixture with the air near the surface of the liquid or within the test vessel (NFPA).
combustible → zapaljiv
The term combustible is often used to describe any material which will burn. However, conditions at which combustion will occur must be defined more accurately, for example a combustible liquid is 37.8 °C but below 93.3 °C. This allows a distinction to be made between combustible materials, which are fairly difficult to ignite, and flammable or highly flammable ones, which are far easier to ignite.
critical point → kritična točka
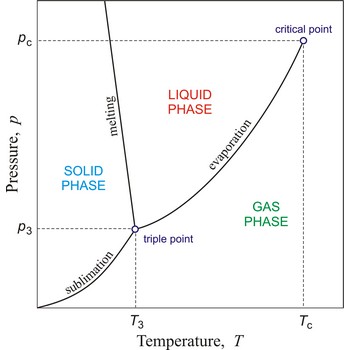
In general, critical point is the point on the phase diagram of a two-phase system at which the two coexisting phases have identical properties and therefore represent a single phase. At the liquid-gas critical point of a pure substance, the distinction between liquid and gas vanishes, and the vapour pressure curve ends. The coordinates of this point are called the critical temperature and critical pressure. Above the critical temperature it is not possible to liquefy the substance.
cryogenic fractionation → kriogena frakcinacija
Cryogenic fractionation is a process of separation of gases by cooling them until they enter their liquid state. Large scale gas production companies use this method to produce liquid oxygen, liquid nitrogen etc. Gases have different boiling points (the temperature at which they change from liquid to gas). Oxygen has a boiling point of -183 °C, and nitrogen a boiling point of -195.8 °C. Therefore by cooling the gas mixture to -183 °C, the oxygen can be collected as liquid and the nitrogen remains its gaseous form.
Dewar flask → Dewarova posuda
Dewar flask or vacuum bottle is a container for storing hot or cold substances. It consists of two flasks, one placed inside the other, with a vacuum between. The vacuum prevents the conduction of heat from one flask to the other. For greater efficiency the flasks are silvered to reflect heat. The substance to be kept hot or cold, e.g., liquid air, is contained in the inner flask. The flask is named after British chemist and physicist Sir James Dewar (1842-1923). Dewar invented the Dewar flask in 1892 to aid him in his work with liquid gases. The common thermos bottle is an adaptation of the Dewar flask.
fluid mechanics → mehanika fluida
Fluid mechanics is the study of various properties of the fluid (liquids and gases): velocity, pressure, density and temperature, as functions of space and time.
freezing point → ledište
Freezing point is the temperature at which a liquid becomes a solid at normal atmospheric pressure.
See Melting point
glacial acetic acid → ledena octena kiselina
Glacial acetic acid (CH3COOH) is the pure compound, as distinguished from the usual water solutions known as acetic acid. It is a colorless liquid or crystalline substance (melting point 16.6 °C) with a pungent, vinegar odor.
heat of sublimation → toplina sublimacije
Heat of sublimation or enthalpy of sublimation is the energy required to convert one mole of a substance from the solid to the gas state (sublimation) without the appearance of the liquid state.
Citing this page:
Generalic, Eni. "Pothlađena tekućina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table