dipole moment → dipolni moment
Electric dipole moment (μ) is a product of the positive charge and the distance between the charges. Dipole moments are often stated in debyes; The SI unit is the coulomb metre. In a diatomic molecule, such as HCl, the dipole moment is a measure of the polar nature of the bond; i.e. the extent to which the average electron charges are displaced towards one atom (in the case of HCl, the electrons are attracted towards the more electronegative chlorine atom). In a polyatomic molecule, the dipole moment is the vector sum of the dipole moments of the individual bonds. In a symmetrical molecule, such as tetrafluoromethane (CF4) there is no overall dipole moment, although the individual C-F bonds are polar.
electronegativity → elektronegativnost
Electronegativity is a parameter originally introduced by L. Pauling which describes, on a relative basis, the power of an atom to attract electrons. For example, in hydrogen chloride, the chlorine atom is more electronegative than the hydrogen and the molecule is polar, with a negative charge on the chlorine atom.
There are various ways of assigning values for the electronegativity of an element. Pauling electronegativities are based on bond dissociation energies using a scale in which fluorine, the most electronegative element, has the value 4 and francium, the lowest electronegative element, has the value 0.7.
Fajans’ rules → Fajansova pravila
Fajans’ rules, formulated by American chemist of Polish origin. Kazimierz Fajans (1887-1975), indicating the extent to which an ionic bond has covalent character caused by polarisation of the ions. Covalent character is more likely if:
1. the charge of the ions is high;
2. the positive ion is small or the negative ion is large;
3. the positive ion has an outer electron configuration that is not a noble- gas configuration.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
resonance → rezonancija
Resonance is a stabilising quality of certain molecules that can be represented by considering the electron distribution in an ion or molecule as a composite of two or more forms, in those cases where a single form is an inadequate representation; for example, benzene and the carbonate ion. A various canonical structures can be drawn to show how electron delocalisation will explain the discrepancy, the difference in electron density
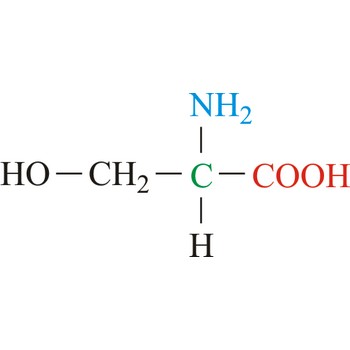
serine → serin
Serine is neutral amino acids with polar side chains. It is one of two hydroxyl amino acids. Both are commonly considered to by hydrophilic due to the hydrogen bonding capacity of the hydroxyl group. Serine often serves as a nucleophile in many enzyme active sites, and is best known for its role in the serine proteases. Serine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine.
- Abbreviations: Ser, S
- IUPAC name: 2-amino-3-hydroxypropanoic acid
- Molecular formula: C3H7NO3
- Molecular weight: 105.09 g/mol
structural formula → strukturna formula
Structural formula is a two dimensional representations of the arrangement of the atoms in molecules. Atoms are represented by their element symbols and covalent bonds are represented by lines. The symbol for carbon is often not drawn.
addition reactions → reakcije adicije
Addition reactions are normally occur with unsaturated compounds and involve the addition of one molecule (called the reactant) across the unsaturated bond (i.e. the double bond or the triple bond) of another molecule (called the substrate) to give a single product, formed by the combination of both reacting molecules.
For example, bromine adds across the double bond of ethene in an addition reaction to form dibromoethane.
Citing this page:
Generalic, Eni. "Polarna kovalentna veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table