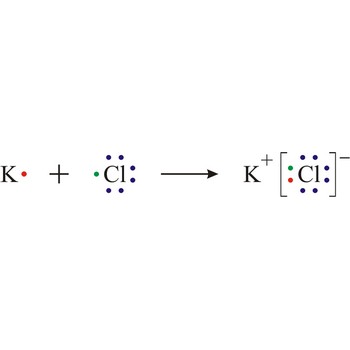ionic bond → ionska veza
Ionic bond is a strong force of attraction holding atoms together in a molecule or crystal. Typically chemical bonds have energies of about 100 kJ mol-1. Ionic bond is a bond at which one of the participants, during the procedure of bonding, gives away its unpaired electrons to another atom so that both can achieve electron arrangement of the closest noble gas. In order to form an ionic bond one of the atoms must cross to the positively charged ion by losing certain number of electrons and the other atom must receive those electrons and cross to the negatively charged ion.
peptide bond → peptidna veza
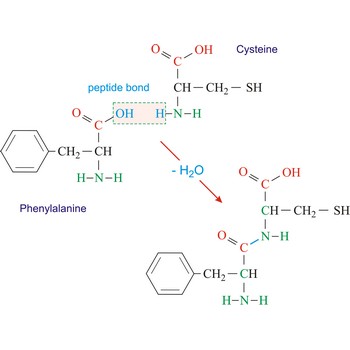
Peptide bond emerges when two amino acid join in a way that the carbon atom from one connects with the nitrogen atom from the other (creating a C-N bond).
polar molecule → polarna molekula
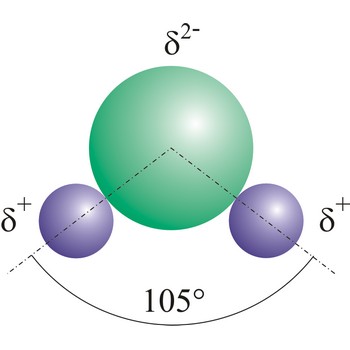
Polar molecules are molecules at which centres of gravity of positive and negative charge are not in the same point.
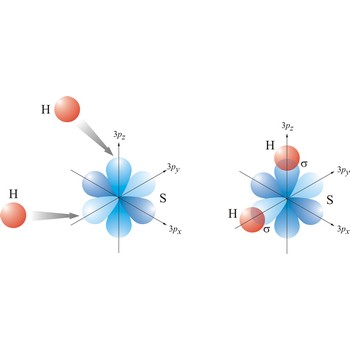
sigma bond → sigma veza
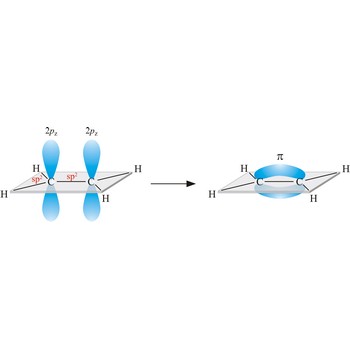
Most single bonds are sigma bonds (σ-bond). In the valence bond theory, a sigma bond is a valence bond that is symmetrical around the imaginary line between the bonded atoms.
covalent compound → kovalentni spoj
Covalent compound is a compound made of molecules - not ions, such as H2O, CH4, Cl2. The atoms in the compound are bound together by shared electrons. Also called a molecular compound.
covalentity → kovalentnost
Covalentity is a maximum number of covalent bond that one atom can make, it is equal to the number of hydrogen atoms that are combined with other atoms. It is usually constant.
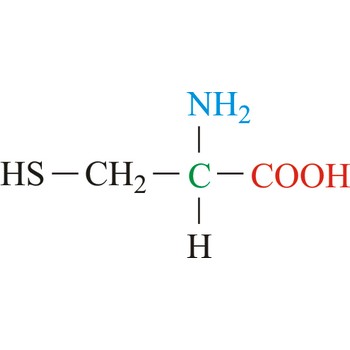
cysteine → cistein
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
polar solvent → polarno otapalo
Polar solvent is a liquid with polar molecules which dissolves polar compounds, because the charges on the endings of the solvent’s molecules attract the elements from ion crystals.
Citing this page:
Generalic, Eni. "Polarna kovalentna veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table