hydrophobic interaction → hidrofobne interakcije
Hydrophobic interaction is the tendency of hydrocarbons (or of lipophilic hydrocarbon-like groups in solutes) to form intermolecular aggregates in an aqueous medium, and analogous intramolecular interactions. The name arises from the attribution of the phenomenon to the apparent repulsion between water and hydrocarbons. Use of the misleading alternative term hydrophobic bond is discouraged.
ion exchanger → ionski izmjenjivač
Ion-exchanger is a solid or liquid material containing ions that are exchangeable with other ions with a like charge that are present in a solution in which the material is insoluble. Ion-exchange resins consist of various copolymers having a cross-linked three-dimensional structure to which ionic groups have been attached.
Kjeldahl’s method → Kjeldahlov postupak
Kjeldahl’s method is an analytical method for determination of nitrogen in certain organic compounds. The method was developed by the Danish chemist Johan Kjeldahl (1849-1900).
It involves addition of a small amount of anhydrous potassium sulphate to the test compound, followed by heating the mixture with concentrated sulphuric acid, often with a catalyst such as copper sulphate. As a result ammonia is formed. After alkalyzing the mixture with sodium hydroxyde, the ammonia is separated by distillation, collected in standard acid, and the nitrogen determined by back-titration.
- Kjeldahl flask for decomposition (500 ml – macro or 100 ml - micro)
- funnel for alkaline solution
- Wagner tube (drop catcher)
- condenser
- absorption flask with known volume of standard acid
lactose → laktoza
Lactose (milk sugar) is a disaccharide comprising one glucose molecule linked to a galactose molecule by an β(1→4)-glycosidic linkage. Lactose has a beta acetal. Lactose is manufactured by the mammary gland and occurs only in milk (from 4 % to 7 %). Lactose intolerance is a common medical condition that results in diarrhea, abdominal pain, and flatulence and is caused by reduced or absent activity of enzyme lactase.
Like cellobiose and maltose, lactose is a reducing sugar. All reducing sugar undergo mutarotation in aqueous solution. The equilibrium mixture at 20 °C is composed of 62.7 % β-lactose (β-D-galactopyranosyl-(1→4)-β-D-glucopyranose) and 37.3 % α-lactose (β-D-galactopyranosyl-(1→4)-α-D-glucopyranose).
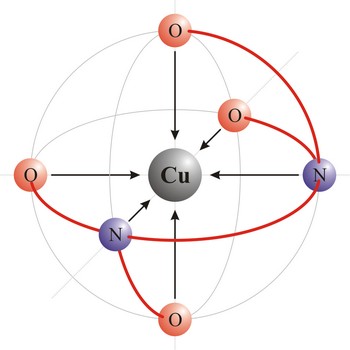
ligand → ligand
Ligand is an ion (F-, Cl-, Br-, I-, S2-, CN-, NCS-, OH-, NH2-) or molecule (NH3, H2O, NO, CO) that donates a pair of electrons to a metal atom or ion in forming a coordination complex. The main way of classifying ligands is by the number of points at which they are attached to, or bound to, the metal center. This is the denticity. Ligands with one potential donor atom are monodentate. Polydentate ligand is a ligand that is attached to a central metal ion by bonds from two or more donor atoms. Ligands with more than one potential donor atom are known as ambidentate, such as the thiocyanate ion, NCS-, which can bind to the metal center with either the nitrogen or sulphur atoms. Chelating ligands are those polydentate ligands which can form a ring including the metal atom.
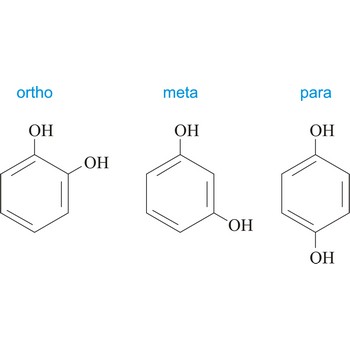
meta position → meta položaj
Meta position in organic chemistry is the one in which there are two same functional groups tied to a ring of benzene in position 1 and 3. The abbreviation m- is used, for example, m-Hydroquinone is 1,3-dihydroxybenzene.
methionine → metionin
Methionine is neutral amino acids with polar side chains. It is one of the two sulfur-containing amino acids. Methionine is a fairly hydrophobic amino acid and typically found buried within the interior of a protein. It can form stacking interactions with the aromatic moieties of tryptophan, phenylalanine, and tyrosine. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Met, M
- IUPAC name: 2-amino-4-methylsulfanylbutanoic acid
- Molecular formula: C5H11NO2S
- Molecular weight: 149.21 g/mol
Newman’s projection → Newmanova projekcija
Newman’s projection is an image which we get when we observe a model of ethane molecule in C-C bond direction.
nitrogen → dušik
Nitrogen was discovered by Daniel Rutherford (Scotland) in 1772. The origin of the name comes from the Greek words nitron genes meaning nitre and forming and the Latin word nitrum (nitre is a common name for potassium nitrate, KNO3). It is colourless, odourless, generally inert gas. Minimally reactive at room temperature. A component of many organic and inorganic compounds. Makes up about 78 % of earth’s atmosphere. Nitrogen is obtained from liquid air by fractional distillation. Primarily to produce ammonia and other fertilizers. Also used in making nitric acid, which is used in explosives. Also used in welding and enhanced oil recovery.
Citing this page:
Generalic, Eni. "Polarna kovalentna veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table