equation of state → jednadžba stanja
Equation of state is an equation relating the pressure, volume, and temperature of a substance or system. Equation of state for ideal gas
where p is pressure, V molar volume, T temperature, and R the molar gas constant (8.314 JK-1mol-1).
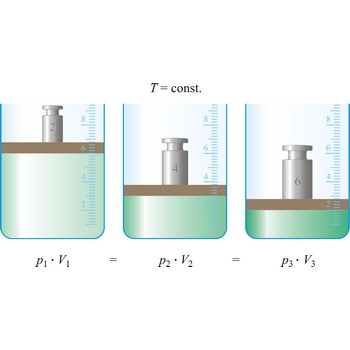
Boyle’s law → Boyleov zakon
Boyle’s law (sometimes referred to as the Boyle-Mariott’s law) is the empirical law, exact only for an ideal gas, which states that the volume of a gas is inversely proportional to its pressure at constant temperature.
carbon → ugljik
Carbon has been known since ancient times. The origin of the name comes from the Latin word carbo meaning charcoal. Graphite form of carbon is a black, odourless, slippery solid. Graphite sublimes at 3825 °C. Diamond form is a clear or colored; an extremely hard solid. C60 is Buckminsterfullerine. Carbon black burns readily with oxidants. Carbon is made by burning organic compounds with insufficient oxygen. There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes. Radiocarbon dating uses the carbon-14 isotope to date old objects.
Carnot cycle → Carnotov kružni proces
Carnot cycle is the most efficient cycle of operations for a reversible heat engine. Published in 1824 by French physicist Nicolas Léonard Sadi Carnot (1796-1832), it consists of four operations on the working substance in the engine:
1-2: Isothermal expansion at thermodynamic temperature T1 with heat QH taken in.
2-3: Adiabatic expansion with a fall of temperature to T2.
3-4: Isothermal compression at temperature T2 with heat QC given out.
4-1: Adiabatic compression at temperature back to T1.
According to the Carnot principle, the efficiency of any reversible heat engine depends only on the temperature range through which it works, rather than the properties of the working substances.
fluid mechanics → mehanika fluida
Fluid mechanics is the study of various properties of the fluid (liquids and gases): velocity, pressure, density and temperature, as functions of space and time.
freezing point → ledište
Freezing point is the temperature at which a liquid becomes a solid at normal atmospheric pressure.
See Melting point
heat of hydration → toplina hidratacije
Heat of hydration or enthalpy of hydration of ions corresponds to the heat that is released by hydration of one mole of ions at a constant pressure. The more the ion is hydrated, the more heat is released. Degree of hydration depends on the size and charge of ion. The smaller the ion and the greater its charge, it will be the more hydrated.
Charles’ law → Charlesov zakon
The volume of a fixed mass of gas at a constant pressure expand by the constant fraction of its volume at 0 °C. For each Celsius or kelvin degree its temperature is raised. For any ideal gas fraction it is approximately 1/273. This can be expressed by the equation
were V° is the volume at 0°C and V is its volume at t°C.
This is equivalent to the statement that the volume of a fixed mass of gas at a constant pressure is proportional to its thermodynamic temperature
This law also know as Gay-Lussac’s law.
An equation similar to the one given above applies to pressures for ideal gases:
Clapeyron equation → Clapeyronova jednadžba
Clapeyron equation (also called the Clausius-Clapeyron equation) is a relation between pressure and temperature of two phases of a pure substance that are in equilibrium,
where ΔtrsS is the difference in entropy between the phases and ΔtrsV the corresponding difference in volume.
Citing this page:
Generalic, Eni. "Parcijalni tlak." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table