energy → energija
Energy (E, U) is the characteristic of a system that enables it to do work. Like work itself, it is measured in joules (J).
The internal energy of a body is the sum of the potential energy and the kinetic energy of its component atoms and molecules.
Potential energy is the energy stored in a body or system as a consequence of its position, shape, or state (this includes gravitation energy, electrical energy, nuclear energy, and chemical energy).
Kinetic energy is the energy of motion and is usually defined as the work that will be done by a body possessing the energy when it is brought to rest. For a body of mass m having a speed v, the kinetic energy is mv2/2. Kinetic energy is most clearly exhibited in gases, in which molecules have much greater freedom of motion than in liquids and solids.
In an isolated system energy can be transferred from one form to another but the total energy of the system remains constant.
extensive property → ekstenzivno svojstvo
Extensive property is a property that changes when the amount of matter in a sample changes. Examples are mass, volume, length, and charge.
fermentation → fermentacija
Fermentation is a class of biochemical reactions that break down complex organic molecules (such as carbohydrates) into simpler materials (such as ethanol, carbon dioxide, and water). Fermentation reactions are catalyzed by enzymes.
Fermi level → Fermijev nivo
Fermi level is the highest energy of occupied states in a solid at zero temperature. The Fermi level in conductors lies in the conduction band, in insulators it lies in the valence band, and in semiconductors it falls in the gap between the conduction band and the valence band. It was named after the Italian physicst Enrico Fermi (1901 - 1954).
enzyme → enzim
Enzyme is a protein that acts as a catalyst in biochemical reactions. Each enzyme is specific to a particular reaction or a group of similar reactions. Many require the association of certain nonprotein cofactors in order to function. The molecule undergoing a reaction (the substrate) binds to a specific active site on the enzyme molecule to form a short-lived intermediate: this greatly increases (by a factor of up to 1020) the rate at which the reaction proceeds to form the product.
extraction → ekstrakcija
Extraction is the separation of a component from its mixture by selective solubility. When a solution of one substance in one solvent is brought in with another solvent dissolved substance will distribute between the two solutants because of different solubility. Extraction is an efficient and fast method used for separating and concentrating matters. Extraction is best done several times in a succession, with smaller amount of solvent in it the matter is better dissolved. For example, caffeine can be separated from coffee beans by washing the beans with supercritical fluid carbon dioxide; the caffeine dissolves in the carbon dioxide, but flavour compounds do not. Vanillin can be extracted from vanilla beans by shaking the beans with an organic solvent, like ethanol.
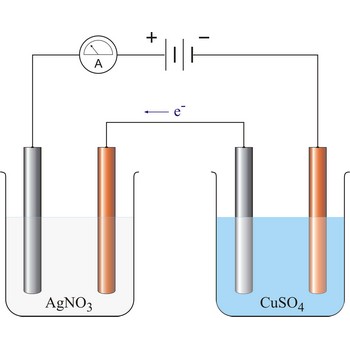
Faraday’s laws of electrolysis → Faradayevi zakoni elektrolize
Faraday’s laws of electrolysis are two laws found by British chemist and physicist Michael Faraday (1791-1867) in his experiments on electrolysis:
1. The quantity of matter extracted on the electrode is proportional to the quantity of charge (Q = I·t) which has flown in electrolysis time.
where z = number of electrons changed in reaction and F = Faraday’s constant which equals 96 487 C mol-1.
2. The masses of the elements liberated by the same quantity of electricity are directly proportional to their chemical equivalents.
96 487 C will discharge 1 mol Ag and 1/2 mol Cu. The relevant half reactions are:
Fick’s law → Fickov zakon
Fick’s law is the statement that the flux J of a diffusing substance is proportional to the concentration gradient, i.e.,
where D is called the diffusion coefficient.
ferromagnetism → feromagnetizam
Ferromagnetism is a type of magnetism in which the magnetic moments of atoms in a solid are aligned within domains which can in turn be aligned with each other by a weak magnetic field. The total magnetic moment of a sample of the substance is the vector sum of the magnetic moments of the component domains. In an unmagnetized piece of ferromagnetic material the magnetic moments of the domains themselves are not aligned; when an external field is applied those domains that are aligned with the field increase in size at the expense of the others. Ferromagnetic materials can retain their magnetisation when the external field is removed, as long as the temperature is below a critical value, the Curie temperature. They are characterised by a large positive magnetic susceptibility.
filter paper → filtar papir
Filter paper is a quantitative paper used for filtering and made of pure cellulose treated with hydrochloric and hydrofluoric acid. This kind of paper burns out practically without any remains (less than 0.0001 g ashes). Different types of paper are marked with numbers; qualitative bears marking 595 or 597 and quantitative 589 or 590. Dependable upon precipitate character, different types of filter paper are used:
- black band (5891) - 100 mL of fluid flows through it in 20 s to 30 s. It is used for filtering of gelatinous precipitates.
- white band (5892) - 100 mL of fluid flows through it in 40 s to 60 s. It is used for coarse crystalline precipitates filtration.
- blue band (5893) - 100 mL of fluid flows through it in 200 s to 400 s. It is used for fine crystalline precipitates.
Citing this page:
Generalic, Eni. "Označena tvar." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table