reactivity → reaktivnost
Reactivity is the tendency of a compound to chemically react with other substances or itself, resulting in the release of energy.
Kjeldahl’s method → Kjeldahlov postupak
Kjeldahl’s method is an analytical method for determination of nitrogen in certain organic compounds. The method was developed by the Danish chemist Johan Kjeldahl (1849-1900).
It involves addition of a small amount of anhydrous potassium sulphate to the test compound, followed by heating the mixture with concentrated sulphuric acid, often with a catalyst such as copper sulphate. As a result ammonia is formed. After alkalyzing the mixture with sodium hydroxyde, the ammonia is separated by distillation, collected in standard acid, and the nitrogen determined by back-titration.
- Kjeldahl flask for decomposition (500 ml – macro or 100 ml - micro)
- funnel for alkaline solution
- Wagner tube (drop catcher)
- condenser
- absorption flask with known volume of standard acid
lead → olovo
Lead has been known since ancient times. The origin of the name comes from the Latin word plumbum meaning liquid silver. It is very soft, highly malleable and ductile, blue-white shiny metal. Tarnishes in moist air; stable in oxygen and water. Dissolves in nitric acid. Compounds toxic by inhalation or ingestion. Danger of cumulative effects. Lead is found most often in ores called galena or lead sulfide (PbS). Used in solder, shielding against radiation and in batteries.
mineral → mineral
Minerals are compounds in which metals can be found in nature. Metals in nature can appear as:
| autochthonous | Au, Cu, Pt, Ag, Pd, Hg, Ir |
| oxides | Fe, Al, Sn, Cr, Mn, W, Cu |
| sulphides | Cu, Pb, Zn, Ni, Ag, Co, Sb, Hg, Mo, Cd, Bi |
| carbonates | Fe, Zn, Cu, Mg, Mn, Pb |
| silicates | Ni, Cu, Zn, Mn |
| chlorides | Ag, Cu, Mg, Na, K |
| sulphates | Ca, Ba, Sr, Cu |
Schiff base → Schiffova baza
Schiff base is a class of compounds derived by the chemical reaction (condensation) of aldehydes or ketones with aromatic amines, for example
They were named after the German chemist Hugo Schiff (1834-1915).
monosaccharide → monosaharid
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
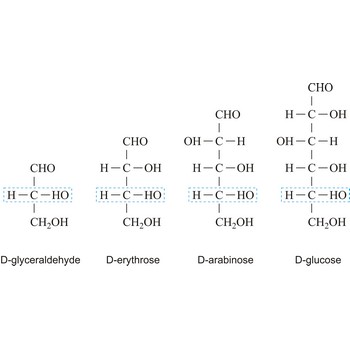
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
nerve poison → živčani bojni otrov
Nerve poison (nerve gas, agents) have had an entirely dominant role since the Second World War. Nerve poisons acquired their name because they affect the transmission of nerve impulses in the nervous system. All nerve poisons belong chemically to the group of organo-phosphorus compounds. They are stable and easily dispersed, highly toxic and have rapid effects both when absorbed through the skin and via respiration. Nerve poisons can be manufactured by means of fairly simple chemical techniques. The raw materials are inexpensive and generally readily available.
The most important nerve agents included in modern chemical weapons arsenals are:
| Tabun | (o-ethyl dimethylamidophosphorylcyanide) |
| Sarin | (isopropyl methylphosphonofluoridate) |
| Soman | (pinacolyl methylphosphonofluoridate) |
| GF | (cyclohexyl methylphosphonofluoridate) |
| VX | (o-ethyl S-diisopropylaminomethyl methylphosphonothiolate) |
Nerve poisons are colorless, odorless, tasteless liquids of low volatility. Antidotes are atropine sulfate and pralidoxime iodide.
Citing this page:
Generalic, Eni. "Oxo compound." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table