osmotic pressure → osmotski tlak
Osmotic pressure (Π) is the excess pressure necessary to maintain osmotic equilibrium between a solution and a pure solvent separated by a membrane permeable only to the solvent. In an ideal dilute solution
where cB is the amount-of-substance concentration of the solute, R is the molar gas constant, and T the temperature.
atmospheric pressure → atmosferski tlak
Atmospheric pressure is the pressure exerted by weight of the air above it at any point on the earth’s surface. At sea level the atmosphere will support a column of mercury about 760 mm high. This decreases with increasing altitude. The standard value for the atmospheric pressure at sea level in SI units is 101 325 Pa.
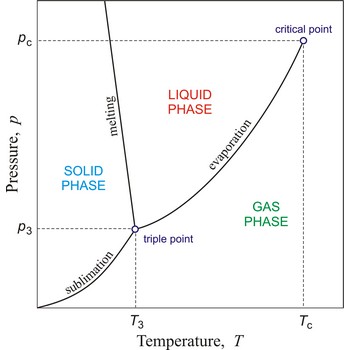
critical pressure → kritični tlak
Critical pressure is the pressure of a fluid in its critical point; i.e. when it is at its critical temperature and critical volume.
partial pressure → parcijalni tlak
Partial pressure is a pressure that one component of gas mixture would have if it were alone in the same volume and at the same temperature as the mixture is in now.
vapour pressure → tlak pare
Vapour pressure is the pressure of a gas in equilibrium with a liquid (or, in some usage, a solid) at a specified temperature.
vapour pressure lowering → snižavanje tlaka pare
Vapour pressure is a colligative property of solutions. The vapour pressure of a solution is always lower than the vapour pressure of the pure solvent. Ratio of solution to pure solvent vapour pressures is approximately equal to the mole fraction of solvent in the solution.
isotonic solution → izotonična otopina
Isotonic solutions are the solutions that have equal osmotic pressure.
osmometry → osmometrija
Osmometry is a determination of the average molecular weight of a dissolved substance from measurements of osmotic pressure.
osmosis → osmoza
Osmosis is the flow of a solvent in a system in which two solutions of different concentration are separated by a semipermeable membrane which cannot pass solute molecules. The solvent will flow from the side of lower concentration to that of higher concentration, thus tending to equalise the concentrations. The pressure that must be applied to the more concentrated side to stop the flow is called the osmotic pressure.
Citing this page:
Generalic, Eni. "Osmotski tlak." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table