krypton → kripton
Krypton was discovered by Sir William Ramsay and Morris W. Travers (England) in 1898. The origin of the name comes from the Greek word kryptos meaning hidden. It is colourless, odourless rare noble gas. Reacts only with fluorine. Krypton is obtained from production of liquid air. Used in lighting products. Some is used as inert filler-gas in incandescent bulbs. Some is mixed with argon in fluorescent lamps. The most important use is in flashing stroboscopic lamps that outline airport runways.
lanthanum → lantan
Lanthanum was discovered by Carl Gustaf Mosander (Sweden) in 1839. The origin of the name comes from the Greek word lanthanein meaning to lie hidden. It is soft, silvery-white, malleable, ductile metal. Readily tarnishes in air. Reaction with water releases hydrogen gas. Metal ignites and burns readily. Reacts with oxidants. Lanthanum is found with rare earths in monazite and bastnasite. Monazite sand typical contains 25 % lanthanum. It is used in the electrodes of high-intensity, carbon-arc lights. Because it gives glass refractive properties, it is used in expensive camera lenses.
lawrencium → lawrencij
Lawrencium was discovered by Albert Ghiorso, Torbjorn Sikkeland, Almon E. Larsh and Robert M. Latimer (USA) in 1961. Named in honour of Ernest O. Lawrence, inventor of the cyclotron. It is synthetic radioactive metal. Lawrencium was produced by bombarding a mixture of three isotopes of californium with boron-10 and boron-11 ions. Eight isotopes of lawrencium have been synthesized to date, with the longest-lived being lawrencium-256, which has a half-life of about 30 seconds.
lead → olovo
Lead has been known since ancient times. The origin of the name comes from the Latin word plumbum meaning liquid silver. It is very soft, highly malleable and ductile, blue-white shiny metal. Tarnishes in moist air; stable in oxygen and water. Dissolves in nitric acid. Compounds toxic by inhalation or ingestion. Danger of cumulative effects. Lead is found most often in ores called galena or lead sulfide (PbS). Used in solder, shielding against radiation and in batteries.
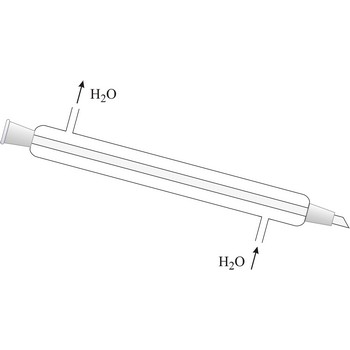
Liebig condenser → Liebigovo hladilo
Liebig condenser is used for condensing of vapours that pass trough the centre tube. It is cooled with water that passes in the outer tube (shell around the centre tube) in the opposite direction than the one of hot vapour. Though named after the German chemist Justus von Liebig (1803-1873), he cannot be given credit for having invented it because it had already been in use for some time before him.
lithium → litij
Lithium was discovered by Johan August Arfvedson (Sweden) in 1817. The origin of the name comes from the Greek word lithos meaning stone, apparently because it was discovered from a mineral source whereas the other two elements, sodium and potassium, were discovered from plant sources. It is soft silvery-white metal. Lightest of metals. Reacts slowly with water and oxygen. Flammable. Can ignite in air. Reacts with water to give off a flammable gas. Lithium is obtained by passing electric charge through melted lithium chloride and from the silicate mineral called spodumene [LiAl(Si2O6)]. Used in batteries. Also for certain kinds of glass and ceramics. Some is used in lubricants.
lutetium → lutecij
Lutetium was discovered by Georges Urbain (France) and independently by Carl Auer von Welsbach (Austria) in 1907. The origin of the name comes from the Greek word Lutetia meaning Paris. It is silvery-white and relatively stable in air, rare earth metal. Lutetium is found with ytterbium in gadolinite and xenotime. Stable lutetium nuclides can be used as catalysts in cracking, alkylation, hydrogenation, and polymerization.
magnesium → magnezij
Magnesium was discovered by Sir Humphry Davy (England) in 1808. The origin of the name comes from the Greek word Magnesia, a district of Thessaly. It is lightweight, malleable, silvery-white metal. Burns in air with a brilliant white flame and reacts with water as temperature elevates. Can ignite in air. React violently with oxidants. Magnesium is found in large deposits in the form of magnesite, dolomite and other minerals. It is usually obtained by electrolysis of melted magnesium chloride (MgCl2) derived from brines, wells and sea water. Used in alloys to make airplanes, missiles and other uses for light metals. Have structural properties similar to aluminium.
manganese → mangan
Manganese was discovered by Johann Gahn (Sweden) in 1774. The origin of the name comes from the Latin word magnes meaning magnet, or magnesia nigri meaning black magnesia (MnO2). It is hard, brittle, grey-white metal with a pinkish tinge. Impure forms are reactive. Rusts like iron in moist air. Manganese is most abundant ores are pyrolusite (MnO2), psilomelane [(Ba,H2O)2Mn5O10] and rhodochrosite (MnCO3). Pure metal produced by mixing MnO2 with powered Al and ignited in a furnace. Used in steel, batteries and ceramics. The steel in railroad tracks can contain as much as 1.2 % manganese. It is crucial to the effectiveness of vitamin B1.
mercury → živa
Mercury has been known since ancient times. The origin of the name comes from the Latin word hydrargyrum meaning liquid silver. It is heavy, silver-white metal, liquid at ordinary temperatures. Stable in air and water. Unreactive with alkalis and most acids. Gives off poisonous vapour. Chronic cumulative effects. Mercury only rarely occurs free in nature. The chief ore is cinnabar or mercury sulfide (HgS). Used in thermometers, barometers and batteries. Also used in electrical switches and mercury-vapour lighting products.
Citing this page:
Generalic, Eni. "Origin of the name Oukonunaka." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table