composition of ocean water → sastav oceanske vode
The proportions of the major constituents of ocean water are almost constant throughout the world. Salinity (total salt content) and the concentrations of individual chemical constituents in sea wateris given the units psu (practical salinity units). For most purposes one can assume that the new unit, psu, and the older unit, ‰, are synonymous.
The average composition of the ocean water is as shown on the following table.
| Constituent | Percentage of total salt |
|---|---|
| Chlorine | 55.3 % |
| Sodium | 30.8 % |
| Magnesium | 3.7 % |
| Sulphur | 2.6 % |
| Calcium | 1.2 % |
| Potassium | 1.1 % |
decomposing → raščinjavanje
Decomposing in analytical chemistry means that a certain substance is converted, by melting it with a suitable melting medium (sodium carbonate, sodium hydroxide, sodium peroxide, ...) in the kind of compound which will afterwards that dissolve in water, acid or base very easily.
diazo compounds → diazo-spojevi
Diazo compounds are compounds having the divalent diazo group, =N+=N-, attached to a carbon atom. The term includes azo compounds, diazonium compounds, and also such compounds as diazomethane, CH2=N2.
contact procedure → kontaktni postupak
Contact procedure is an industrial procedure used for the production of sulphuric acid, where a dry and clean sulphur dioxide and air go over a catalyst made of vanadium pentoxide at 450 °C by which sulphur trioxide is gained, then we add concentrated sulphuric acid by which we obtain smoking sulphuric acid which is now diluted to sulphuric acid.
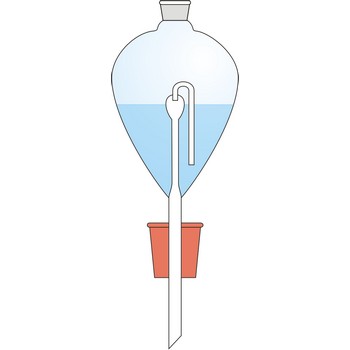
Contat-Gockel’s valve → Contat-Gockelov ventil
Contat-Göckel’s valve is used for maintenance of inert atmosphere in a flask. The valve is filled with a saturated solution of sodium bicarbonate (NaHCO3) so that the end of the tube is covered. Solution inside the valve keeps the flask contents away from the oxygen influence from air. If low pressure is created inside the flask (when the flask is cooled), the solution will penetrate inside it from funnel and in a reaction with acid CO2 is generated which fills up the flask.
Solution from the funnel will keep penetrating until CO2 pressure in the flask is equalised with the outer pressure.
copolymer → kopolimer
Copolymers are also known as heteropolymers. They are made from two (or more) different monomers, which usually undergo a condensation reaction with the elimination of a simple molecule, such as ammonia or water. A typical example is the condensation of 1,6-diaminohexane (hexamethylenediamine) with hexanedioic acid (adipic acid) to form nylon 6,6.
The properties of a polymeric plastic can most easily be modified if it is a copolymer of two or more different monomers, e.g. acrylonitrile-butadiene-styrene copolymer (ABS). Varying the proportions of the component monomers can preselect its properties.
copper → bakar
Copper has been known since ancient times. The origin of the name comes from the Latin word cuprum meaning the island of Cyprus famed for its copper mines. It is malleable, ductile, reddish-brown metal. Resistant to air and water. Exposed surfaces form greenish carbonate film. Pure copper occurs rarely in nature. Usually found in sulfides as in chalcopyrite (CuFeS2), coveline (CuS), chalcosine (Cu2S) or oxides like cuprite (Cu2O). Most often used as an electrical conductor. Also used in the manufacture of water pipes. Its alloys are used in jewellery and for coins.
effervescence → pjenušanje
Effervescence is the formation of gas bubbles in a liquid by a chemical reaction. An example of effervescence is the release of carbon dioxide which bubbles as a gas from the liquid when limestone chips, which are composed of calcium carbonate, are added to dilute hydrochloric acid.
Citing this page:
Generalic, Eni. "Organska kiselina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table