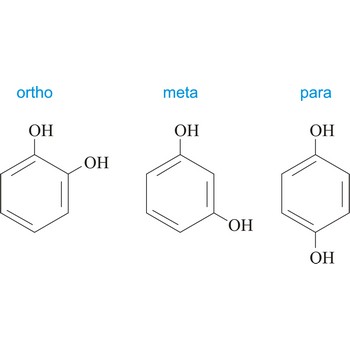
ortho position → ortho položaj
Ortho position in organic chemistry is the one in which there are two same functional groups, tied to a ring of benzene in the positions 1 and 2. The abbreviation o- is used, for example, o-Hydroquinone is 1,2-dihydroxybenzene.
para position → para položaj
Para position in organic chemistry is the one in which there are two same functional groups tied to a ring of benzene in the position 1 and 4. The abbreviation p- is used, for example, p-Hydroquinone is 1,4-dihydroxybenzene.
seawater → more
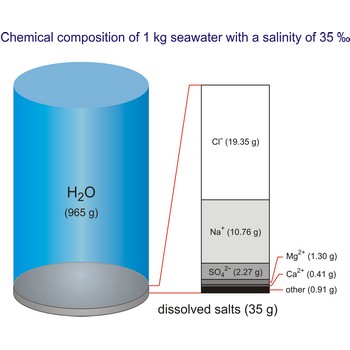
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
sedimentary rocks → sedimentne stijene
Sedimentary Rocks are formed by the accumulation and subsequent consolidation of sediments into various types of rock. There are three major types of sedimentary rocks:
Biogenic sedimentary rocks are formed from organic processes when organisms use materials dissolved in water to build a shell or other skeletal structure.
Clastic sedimentary rocks are composed directly of the sediments or fragments from other rocks.
Chemical sedimentary rocks are formed through evaporation of a chemical rich solution.
Based on their sizes, sediment particles are classified, based on their size, into six general categories:
- boulder (>256 mm)
- cobble (64 - 256 mm)
- gravel (2 - 64 mm)
- sand (1/16 - 2 mm)
- silt (1/256 - 1/16 mm)
- clay (<1/256 mm)
accelerator → akcelerator
Accelerator is a device (machine) used for acceleration of charged particles (protons, deuterons, α-particles). Particles are accelerated under the influence of an electric field and with the help of a magnetic field are kept inside a certain space. When the particles reach enough acceleration (that is sufficient energy), they are directed on a target we wish to bomb. Best known types cyclotron, synchrotron, betatron.
Accelerator is a substance that increases the rate of chemical reaction, i.e. a catalyst.
accumulator → akumulator
Accumulator (secondary cell, storage battery) is a type of voltaic cell or battery that can be recharged by passing current through it from an external D.C. supply. The charging current reverses the chemical reactions in the cell. The common types are the lead-acid accumulator and the nickel-cadmium cell.
acetal → acetal
Acetals are organic compounds having the structure R2C(OR’)2 (R’ ≠ H). They are organic compounds formed by addition of alcohol molecules to aldehyde or ketone molecules. Originally, the term was confined to derivatives of aldehydes (one R = H), but it now applies equally to derivatives of ketones (neither R = H ). Mixed acetals have different R’ groups. The formation of acetals is reversible; acetals can be hydrolysed back to aldehydes (ketone) in acidic solutions.
Acetal, 1,1-diethoxyethane (CH3CH(OC2H5)2), is an organic compound, pleasant smelling, formed by addition of ethyl alcohol to ethanal (acetaldehyde). It is used as a solvent and in synthetic organic chemistry.
acid halide → kiselinski halogenid
Acid halide is organic compound containing the group -COX where X is a halogen atom.
acid-base indicator → kiselo-bazni indikator
Acid-base indicator is a weak acid or weak base, such as litmus, methyl orange or phenolphthalein, which changes colour when it gains or loses an H+ ion.
Citing this page:
Generalic, Eni. "Organska kemija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table