electrochemical cell → elektrokemijski članak
Electrochemical cell is a device that converts chemical energy into electrical energy or vice versa when a chemical reaction is occurring in the cell. It consist of two electronically conducting phases (e.g., solid or liquid metals, semiconductors, etc) connected by an ionically conducting phase (e.g. aqueous or non-aqueous solution, molten salt, ionically conducting solid). As an electric current passes, it must change from electronic current to ionic current and back to electronic current. These changes of conduction mode are always accompanied by oxidation/reduction reactions.
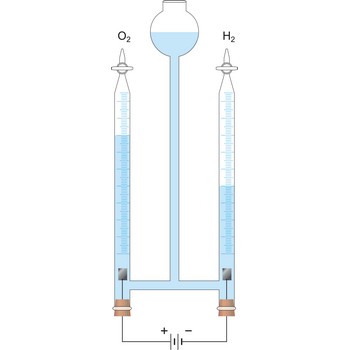
An essential feature of the electrochemical cell is that the simultaneously occurring oxidation-reduction reactions are spatially separated. E.g., in a spontaneous chemical reaction during the oxidation of hydrogen by oxygen to water, electrons are passed directly from the hydrogen to the oxygen.
In contrast, in the spontaneous electrochemical reaction in a galvanic cell the hydrogen is oxidised at the anode by transferring electrons to the anode and the oxygen is reduced at the cathode by accepting electrons from the cathode. The ions produced in the electrode reactions, in this case positive hydrogen ions and the negative hydroxyl (OH-) ions, will recombine in the solution to form the final product of the reaction: water. During this process the electrons are conducted from the anode to the cathode through an outside electric circuit where the electric current can drive a motor, light a light bulb, etc. The reaction can also be reversed: water can be decomposed into hydrogen and oxygen by the application of electrical power in an electrolytic cell.
electrode potential → elektrodni potencijal
Electrode potential is defined as the potential of a cell consisting of the electrode in question acting as a cathode and the standard hydrogen electrode acting as an anode. Reduction always takes place at the cathode, and oxidation at the anode. According to the IUPAC convention, the term electrode potential is reserved exclusively to describe half-reactions written as reductions. The sign of the half-cell in question determines the sign of an electrode potential when it is coupled to a standard hydrogen electrode.
Electrode potential is defined by measuring the potential relative to a standard hydrogen half cell
The convention is to designate the cell so that the oxidised form is written first. For example
The e.m.f. of this cell is
By convention, at p(H2) = 101325 Pa and a(H+) = 1.00, the potential of the standard hydrogen electrode is 0.000 V at all temperatures. As a consequence of this definition, any potential developed in a galvanic cell consisting of a standard hydrogen electrode and some other electrode is attributed entirely to the other electrode
electrodeposition → elektrodepozicija
Electrodeposition is a process of depositing solid materials on an electrode surface using electrolysis. It is a somewhat loosely used term that is applied to many technologies. There are a number of metal deposition technologies. However, not only metals but also different compounds can be electrodeposited. This is used most often for the formation of oxides (such as manganese dioxide and lead dioxide) by anodic oxidation of dissolved salts.
electrolysis → elektroliza
Electrolysis is the decomposition of a substance as a result of passing an electric current between two electrodes immersed in the sample.
Zimmermann-Reinhardt’s reagent → Zimmermann-Reinhardtov reagens
Zimmermann-Reinhardt’s reagent is a mixture of manganese(II) sulphate, sulphuric acid and phosphorus acid. It is used for preventing oxidation of chloride ion while titrating iron(II) ion with permanganate solution.
fossil fuel → fosilno gorivo
Fossil fuels (coal, oil, and natural gas) are the fuels used by man as a source of energy. They are formed from the remains of living organisms and all have a high carbon or hydrogen content. They have value as fuels on the exothermic oxidation of carbon to form carbon dioxide
and the oxidation of hydrogen to form water
Citing this page:
Generalic, Eni. "Oksidacija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table