plutonium → plutonij
Plutonium was discovered by Glenn T. Seaborg, Edwin M. McMillan, J. W. Kennedy and A. C. Wahl (USA) in 1940. Named after the planet Pluto. It is silvery-white, extremely radioactive artificially produced metal. Reacts with oxygen and acids; resists alkalis. Attacked by steam. Highly toxic. Plutonium is found rarely in some uranium ores. Made by bombarding uranium with neutrons. Used in bombs and reactors. Small quantities are used in thermo-electric generators.
polonium → polonij
Polonium was discovered by Marie Curie (Poland) in 1898. Named for Poland, native country of Marie Curie. It is silvery-grey, extremely rare, radioactive metal. Soluble in dilute acids. Highly toxic. Severe radiotoxicity. Carcinogen. Polonium occurs in pitchblende. Produced by bombarding bismuth with neutrons. Used in industrial equipment that eliminates static electricity caused by such processes as rolling paper, wire and sheet metal.
polystyrene → polistiren
Polystyrene is a vinyl polymer. Structurally, it is a long hydrocarbon chain, with a phenyl group attached to every other carbon atom. Polystyrene is produced by free radical vinyl polymerization, from the monomer styrene. Polystyrene or Styrofoam is used in the construction industry as insulating material and for production of containers.
potentiometric titration → potenciometrijska titracija
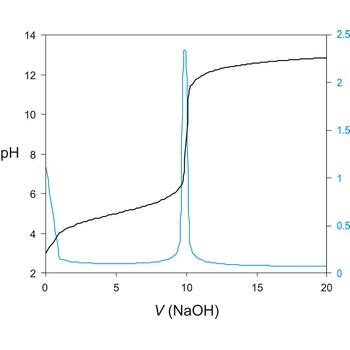
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
protactinium → protaktinij
Protactinium was discovered by Otto Hahn (Germany) and Lise Meitner (Austria) in 1917. The origin of the name comes from the Greek word protos meaning first. It is very rare, silvery-white, extremely radioactive metal. Resists alkalis; reacts with oxygen and acids. Attacked by steam. Highly radiotoxic. Protactinium is extremely toxic and must be handled with great care. Protactinium does not occur in nature. Found among fission products of uranium, thorium and plutonium.
qualitative analysis → kvalitativna analiza
Qualitative analysis involves determining the nature of a pure unknown compound or the compounds present in a mixture. Qualitative inorganic analysis is used to separate and detect cations and anions in a sample substance. According to their properties, cations are usually classified into six groups. Each group has a common reagent which can be used to separate them from the solution.
radium → radij
Radium was discovered by Marie and Pierre Curie (France) in 1898. The origin of the name comes from the Latin word radius meaning ray. It is silvery-white radioactive metal. Reacts with oxygen and water. Highly radiotoxic. Carcinogen by inhalation, ingestion, or exposure. Radium is found in uranium ores at 1 part per 3 million parts uranium. Used in treating cancer because of the gamma rays it gives off.
rutherfordium → ruthefordij
Rutherfordium was discovered by workers at the Nuclear Institute at Dubna (USSR) and by workers at the University of California, Berkeley (USA) in 1964. Name in honour of Lord Rutherford, the physicist and chemist from New Zealand. It is synthetic radioactive metal. Rutherfordium was made by bombarding californium-249 with beams of carbon-12 and 13. Six isotopes of rutherfordium have so far been identified. Rutherfordium-261, the longest-lived, has a half-life of 62 seconds.
saturated fatty acid → zasićena masna kiselina
Saturated fatty acid is a fatty acid carrying the maximum possible number of hydrogen atoms (It doesn’t have any double bounds in the alkyl chain). The most important of these are:
| Butyric (butanoic acid) | CH3(CH2)2COOH |
| Lauric (dodecanoic acid) | CH3(CH2)10COOH |
| Myristic (tetradecanoic acid) | CH3(CH2)12COOH |
| Palmitic (hexadecanoic acid) | CH3(CH2)14COOH |
| Stearic (octadecanoic acid) | CH3(CH2)16COOH |
| Arachidic (eicosanoic acid) | CH3(CH2)18COOH |
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
Citing this page:
Generalic, Eni. "Određivanje starosti radioaktivnim ugljikom." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table