logarithmic scale → logaritamska skala
Logarithmic scale is the one in which values of 1, 2, 3, 4, 5, in fact represents values of 1, 10, 100, 1 000, 10 000. Logarithmic scales are often used to simplify graphs and tables, where otherwise changes of data at the lower end of the scale would be difficult to distinguish (e.g. a graph axis which would normally have values from 1 - 1 000 000 is shown by values of 1 - 7). An example of a logarithmic scale is the pH scale.
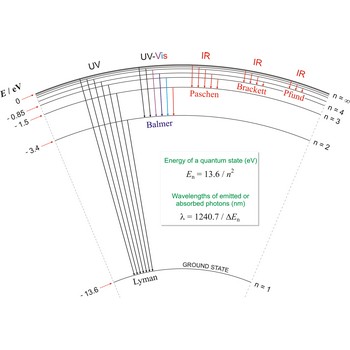
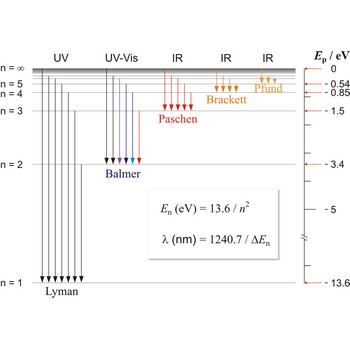
Lyman series → Lymanova serija
Lyman series is the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the ground state (principal quantum number n = 1) and successive excited states.
melting point → talište
Melting point is the temperature at which a solid becomes a liquid at normal atmospheric pressure.
A more specific definition of melting point (or freezing point) is the temperature at which the solid and liquid phases of a substance are in equilibrium at a specified pressure (normally taken to be atmospheric unless stated otherwise). A pure substance under standard condition of pressure has a single reproducible melting point. The terms melting point and freezing point are often used interchangeably, depending on whether the substance is being heated or cooled.
supercooled liquid → pothlađena tekućina
Supercooled liquids are liquids at temperatures below their normal freezing points.
vacuum distillation → vakuum destilacija
Vacuum distillation is distillation under reduced pressure. The depression in the boiling point of the substance distilled means that the temperature is lower, which may prevent the substance from decomposing.
noble gas → plemeniti plin
Noble gas refers to any element of the group of six elements in group 18 of the periodic table. They are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Unlike most elements, the noble gases are monoatomic. The atoms have stable configurations of electrons. Therefore, under normal conditions they do not form compounds with other elements.
They were generally called inert gases until about 1962 when xenon tetrafluoride, XeF4, was produced in the laboratory. This was the first report of a stable compound of a noble gas with another single element.
osmium → osmij
Osmium was discovered by Smithson Tennant (England) in 1803. The origin of the name comes from the Greek word osme meaning smell. It is hard fine black powder or hard, lustrous, blue-white metal. Unaffected by air, water and acids. Characteristic acrid, chlorine like odour due to tetroxide compound. Osmium tetroxide highly toxic. Osmium is obtained from the same ores as platinum. Used to tip gold pen points, instrument pivots, to make electric light filaments. Used for high temperature alloys and pressure bearings. Very hard and resists corrosion better than any other.
Paschen series → Paschenova serija
Paschen series are the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the state with principal quantum number n = 3 and successive higher states.
Citing this page:
Generalic, Eni. "Normalno vrelište." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table