trigonal bipyramidal molecular geometry → trigonska bipiramidalna geometrija molekule
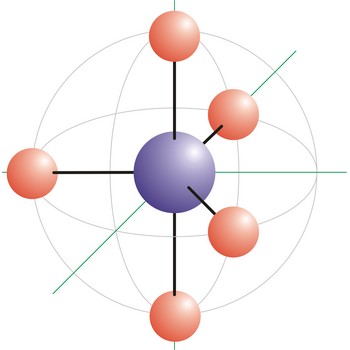
Trigonal bipyramidal (trigonal bipyramidal shape) is a molecular geometry that results when there are five bonds and no lone pairs on the central atom in the molecule. Three of the bonds are arranged along the atom’s equator, with 120° angles between them; the other two are placed at the atom’s axis. Axial bonds are at right angles to the equatorial bonds. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. The PCl5 molecule has a trigonal bipyramidal molecular geometry.
trigonal planar molecular geometry → trigonska planarna geometrija molekule
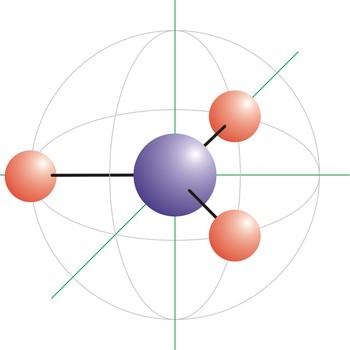
Trigonal planar is a molecular shape that results when there are three bonds and no lone pairs around the central atom in the molecule. The pairs are arranged along the central atom’s equator, with 120° angles between them. Molecules with an trigonal planar electron pair geometries have sp2d hybridization at the central atom. The carbonate ion (CO32-) has a trigonal planar geometry.
trigonal pyramidal molecular geometry → trigonska piramidalna geometrija molekule
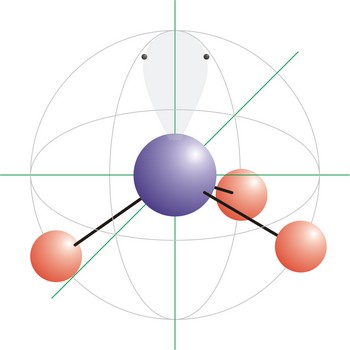
Trigonal pyramidal is a molecular shape that results when there are three bonds and one lone pair on the central atom in the molecule. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. Ammonia (NH3) is a trigonal pyramidal molecule.
alanine → alanin
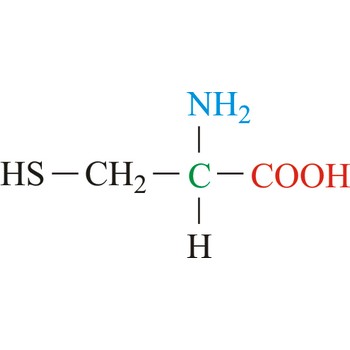
Alanine is hydrophobic amino acids with aliphatic side chain. It is the second simplest amino acid, but used the most in proteins. The nonpolar hydrophobic amino acids tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Alanine is a nonessential amino acid, meaning it can be manufactured by the human body, and does not need to be obtained directly through the diet.
- Abbreviations: Ala, A
- IUPAC name: 2-aminopropanoic acid
- Molecular formula: C3H7NO2
- Molecular weight: 89.09 g/mol
cysteine → cistein
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
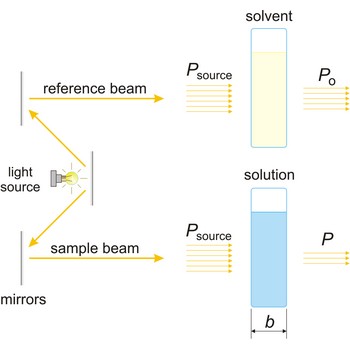
absorbance → apsorbancija
Absorbance (A) is a logarithm of the ratio of incident radiant power (Po) to transmitted radiant power (P) through a sample (excluding the effects on cell walls).
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
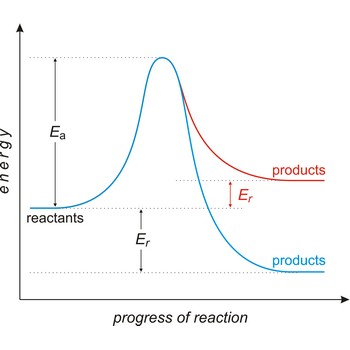
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
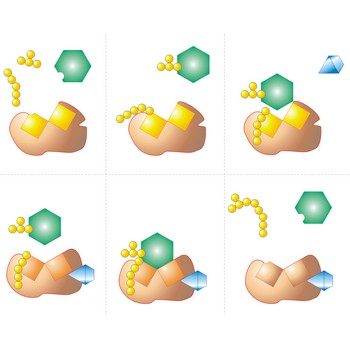
active site → aktivno mjesto
Active site is a pocket or crevice on an enzyme molecule that fits reactant molecules like a hand in a glove. The active site lowers the activation energy for reaction
AMU → AMU
AMU or atomic mass unit is a unit of mass used to express relative atomic masses. It is equal to 1/12 of the mass of an atom of the isotope carbon-12 and is equal to 1.66 033×10-27 kg. This unit superseded both the physical and a chemical mass unit based on oxygen-16 and is sometimes called the unified mass unit or the dalton.
Citing this page:
Generalic, Eni. "Nepolarna molekula." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table