eutectic → eutektik
Eutectic is a solid solution consisting of two or more substances and having the lowest freezing point of any possible mixture of these components.
Eutectic point is the lowest temperature at which the eutectic mixture can exist in a liquid phase. A liquid having the eutectic composition will freeze at a single temperature without a change of composition.
extensive property → ekstenzivno svojstvo
Extensive property is a property that changes when the amount of matter in a sample changes. Examples are mass, volume, length, and charge.
fermentation → fermentacija
Fermentation is a class of biochemical reactions that break down complex organic molecules (such as carbohydrates) into simpler materials (such as ethanol, carbon dioxide, and water). Fermentation reactions are catalyzed by enzymes.
Fermi level → Fermijev nivo
Fermi level is the highest energy of occupied states in a solid at zero temperature. The Fermi level in conductors lies in the conduction band, in insulators it lies in the valence band, and in semiconductors it falls in the gap between the conduction band and the valence band. It was named after the Italian physicst Enrico Fermi (1901 - 1954).
extraction → ekstrakcija
Extraction is the separation of a component from its mixture by selective solubility. When a solution of one substance in one solvent is brought in with another solvent dissolved substance will distribute between the two solutants because of different solubility. Extraction is an efficient and fast method used for separating and concentrating matters. Extraction is best done several times in a succession, with smaller amount of solvent in it the matter is better dissolved. For example, caffeine can be separated from coffee beans by washing the beans with supercritical fluid carbon dioxide; the caffeine dissolves in the carbon dioxide, but flavour compounds do not. Vanillin can be extracted from vanilla beans by shaking the beans with an organic solvent, like ethanol.
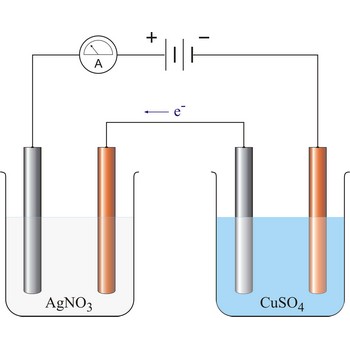
Faraday’s laws of electrolysis → Faradayevi zakoni elektrolize
Faraday’s laws of electrolysis are two laws found by British chemist and physicist Michael Faraday (1791-1867) in his experiments on electrolysis:
1. The quantity of matter extracted on the electrode is proportional to the quantity of charge (Q = I·t) which has flown in electrolysis time.
where z = number of electrons changed in reaction and F = Faraday’s constant which equals 96 487 C mol-1.
2. The masses of the elements liberated by the same quantity of electricity are directly proportional to their chemical equivalents.
96 487 C will discharge 1 mol Ag and 1/2 mol Cu. The relevant half reactions are:
Fick’s law → Fickov zakon
Fick’s law is the statement that the flux J of a diffusing substance is proportional to the concentration gradient, i.e.,
where D is called the diffusion coefficient.
flash point → temperatura zapaljenja
Flash point is the lowest temperature at which a liquid or volatile solid gives off vapour sufficient to form an ignitable mixture with the air near the surface of the liquid or within the test vessel (NFPA).
flotation → flotacija
Flotation is a procedure in which hydrophobic solid substances are separated from hydrophilic one using bubbles of air. If air is blow through a suspension, in which substances promoting easier creation of foam are added, bubbles of air are created which stick to the hydrophobic matter and carry it out to the surface.
Citing this page:
Generalic, Eni. "Mješljiva tvar." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table