glacial acetic acid → ledena octena kiselina
Glacial acetic acid (CH3COOH) is the pure compound, as distinguished from the usual water solutions known as acetic acid. It is a colorless liquid or crystalline substance (melting point 16.6 °C) with a pungent, vinegar odor.
Glauber’s salt → Glauberova sol
Glauber’s salt is sodium sulphate decahydrate (Na2SO4·10H2O). Loses water of hydration at 100 °C. Energy storage capacity is more than seven times that of water.
calomel electrode → kalomel elektroda
Calomel electrode is a type of half cell in which the electrode is mercury coated with calomel (Hg2Cl2) and the electrolyte is a solution of potassium chloride and saturated calomel. In the calomel half cell the overall reaction is
Table: Dependence of potential of calomel electrode upon temperature and concentration of KCl according to standard hydrogen electrode
| Potential vs. SHE / V | |||
|---|---|---|---|
| t / °C | 0.1 mol dm-3 | 3.5 mol dm-3 | sat. solution |
| 15 | 0.3362 | 0.254 | 0.2511 |
| 20 | 0.3359 | 0.252 | 0.2479 |
| 25 | 0.3356 | 0.250 | 0.2444 |
| 30 | 0.3351 | 0.248 | 0.2411 |
| 35 | 0.3344 | 0.246 | 0.2376 |
carbon → ugljik
Carbon has been known since ancient times. The origin of the name comes from the Latin word carbo meaning charcoal. Graphite form of carbon is a black, odourless, slippery solid. Graphite sublimes at 3825 °C. Diamond form is a clear or colored; an extremely hard solid. C60 is Buckminsterfullerine. Carbon black burns readily with oxidants. Carbon is made by burning organic compounds with insufficient oxygen. There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes. Radiocarbon dating uses the carbon-14 isotope to date old objects.
honey → med
Honey is a sweet, amber colored, viscous fluid produced by honeybees from the nectar of flowers. It is composed primarily of fructose (about 40 %), glucose (about 35 %), and water (up to 20 %). In addition, honey contains sucrose, maltose, trisaccharides, and small amounts of minerals, vitamins, and enzymes.
horse power → konjska snaga
Horse power is an obsolete non-SI of power introduced by James Watt in 1782 to allow describing the power of steam machinery. It was equal to the work effort of a horse needed to raise vertically 528 cubic feet of water to one metre in one minute (HP = 735.498750 W).
carbohydrate → ugljikohidrat
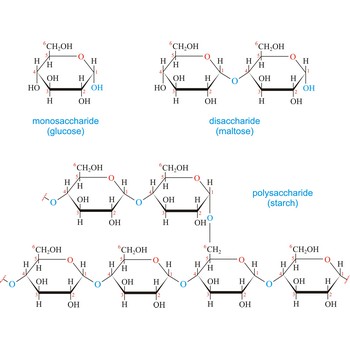
Carbohydrates (often called carbs for short) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. They are also known as saccharides, a term derived from the Latin word saccharum for sugar. Carbohydrates are the most abundant class of compounds in the biological world, making up more than 50 % of the dry weight of the Earth’s biomass. Every type of food we eat can have its energy traced back to a plant. Plants use carbon dioxide and water to make glucose, a simple sugar, in photosynthesis. Other carbohydrates such as cellulose and starch are made from the glucose. Light from the sun is absorbed by chlorophyll and this is converted to the energy necessary to biosynthesize carbohydrates
The term carbohydrate was applied originally to monosaccharides, in recognition of the fact that their empirical composition can be expressed as Cx(H2O)y. Later structural studies revealed that these compounds were not hydrates but the term carbohydrate persists.
Carbohydrates are generally classed as either simple or complex. Simple sugars, or monosaccharides, are carbohydrates that can’t be converted into smaller subunits by hydrolysis. Complex carbohydrates are made of two (disaccharides) or more (oligosaccharides, polysaccharides) simple sugars linked together by acetal (glycosidic) bonds and can be split into the former by hydrolysis.
cellulose → celuloza
Cellulose, (C6H10O5)n, is a polysaccharide that consists of a long unbranched chain of glucose units linked by (1→4)-β-glycoside bonds. Nature uses cellulose primarily as a structural material to impart strength and rigidity to plants. Leaves, grasses, and cotton are primarily cellulose. The fibrous nature of extracted cellulose has led to its use in textile industry for the production of cotton, artificial silk, etc. Cellulose also serves as raw material for the manufacture of cellulose acetate, known commercially as acetate rayon, and cellulose nitrate, known as guncotton. Gunncotton is the major ingredient in smokeless powder, the explosive propellant used in artillery shells and in ammunition for firearms.
Citing this page:
Generalic, Eni. "Meka voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table