EDTA → EDTA
Ethyldiaminetetraacetic acid (C10H16N2O8) or shortened EDTA is a hexadentant ligand, and it forms chelates with both transition-metal ions and main-group ions. EDTA is used as a negative ion - EDTA4-. The diagram shows the structure of the ion with the important atoms picked out. The EDTA ion entirely wraps up a metal ion using all 6 of the positions. The co-ordination number is again 6 because of the 6 co-ordinate bonds being formed by the central metal ion.
EDTA is frequently used in soaps and detergents, because it forms a complexes with calcium and magnesium ions. These ions are in hard water and interfere with the cleaning action of soaps and detergents. EDTA is also used extensively as a stabilizing agent in the food industry and as an anticoagulant for stored blood in blood banks. EDTA is the most common reagent in complexometric titration.
measuring cylinder → menzura
Measuring cylinders (graduated cylinders) are graduated glass cylinders with a capacity from 2 mL to 2 L. They are used when reagent solutions for volume measuring are prepared when accuracy is not of great relevance. The larger the measuring cylinder, the bigger the measuring error.
volumetry → volumetrija
Volumetry consists of adding an equivalent amount of a reagent of exactly known concentration to the analyte. From stechiometrical proportion and added volume of reagent the quantity of matter in question can be calculated.
pipette → pipeta
Pipettes are glass tubes which are tapers towards at both ends into narrow opened tubes. According to their design two types of pipettes can be distinguished:
Volumetric pipettes
Volumetric pipettes (transfer or belly pipette) are used in volumetric analysis, when there is a need for taking exact smaller volume of a sample solution or reagent. The upper tube of volumetric pipette has a ringlike marking (mark) which marks its calibrated volume. Pipettes calibrated to deliver (TD or Ex) the indicated volume. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark. Mark must figure as a tangent on a lower edge of the liquid meniscus. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely. After another 15 s and the tip of the pipette is pulled onto the inner wall of the vessel. It is absolutely forbidden to blow out the contents of the pipette
Graduated pipettes
Graduated pipettes (Mohr pipette) have a scale divided into units of one and of 1/10th of a millilitre. Because of their wide necks it is less accurate than the volumetric pipette. They are used when taking volume of solutions in which accuracy does not have to be very high. They are filled in the same way as volumetric ones and liquid can be gradually released.
potentiometric titration → potenciometrijska titracija
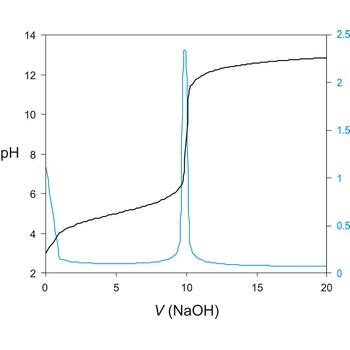
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
qualitative analysis → kvalitativna analiza
Qualitative analysis involves determining the nature of a pure unknown compound or the compounds present in a mixture. Qualitative inorganic analysis is used to separate and detect cations and anions in a sample substance. According to their properties, cations are usually classified into six groups. Each group has a common reagent which can be used to separate them from the solution.
standard → standard
Standards are materials containing a known concentration of an analyte. They provide a reference to determine unknown concentrations or to calibrate analytical instruments.
The accuracy of an analytical measurement is how close a result comes to the true value. Determining the accuracy of a measurement usually requires calibration of the analytical method with a known standard. This is often done with standards of several concentrations to make a calibration or working curve.
A primary standard is a reagent that is extremely pure, stable, has no waters of hydration, and has a high molecular weight.
A secondary standard is a standard that is prepared in the laboratory for a specific analysis. It is usually standardised against a primary standard.
supercritical fluid extraction → superkritična fluidna ekstrakcija
Supercritical fluid extractions (SFE) have solvating powers similar to liquid organic solvents, but with higher diffusivities, lower viscosity, and lower surface tension. The main advantages of using supercritical fluids for extractions is that they are inexpensive, contaminant free, and less costly to dispose safely than organic solvents. For non-destructive isolation choose SFE, which is simply the best technology for sensitive raw materials. For these reasons supercritical carbon dioxide (scCO2) is the reagent used to extract caffeine from coffee and tea. Its gaslike behavior allows it to penetrate deep into the green coffee beans, and it dissolves from 97 % to 99 % of the caffeine present.
volumetric flask → odmjerna tikvica
Volumetric flasks are bottles made of glass, in a pear like in shape with long thin necks and flat bottoms. All come with a ground glass stopper for a tight seal. Volume marking is cut in glass with fluoride acid around the neck, so that parallax should be avoided (flask is put in front of the eyes so that one can see only a straight horizontal line). A volumetric flask is calibrated to contain (TC or In) the indicated volume of water at 20 °C when the bottom of the meniscus is adjusted to just rest on the center of the line marked on the neck of the flask. They are used for preparing the exactly known volume of sample solution and standard solutions of reagents. On each flask with volume designation a temperature on which the flask has been calibrated is designated.
Citing this page:
Generalic, Eni. "Maskirajući reagens." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table