ceiling level → maksimalno dopustiva koncentracija
Ceiling level or ceiling value is the maximum permissible concentration of a hazardous material in the working environment. This level should not be exceeded at any time.
amount concentration → količinska koncentracija
Amount concentration (also called molar concentration and in older literature molarity) is the amount of a given substance in a stated unit of a mixture, solution, or ore. The common unit is mole per cubic decimetre (moldm−3) or mole per litre (molL-1) sometimes denoted by M.
The concentration of an atom, ion, or molecule in a solution may be symbolised by the use of square brackets, as [Ca2+].
concentration → koncentracija
1. Group of four quantities characterizing the composition of a mixture with respect to the volume of the mixture (mass, amount, volume and number concentration).
2. Short form for amount (of substance) concentration (substance concentration in clinical chemistry).
mass concentration → masena koncentracija
Mass concentration (γ) is equal to mass (mA) of soluted substance and volume (V) of the solution proportion. SI unit for mass concentration is kg m-3, but in laboratory practice g dm-3, which has the same number value, is often used.
volume concentration → volumenska koncentracija
Volume concentration (σ) is equal to volume (VA) of solute and volume (V) of solution proportion. Volume concentration differs from volume fraction because the sum of solution components volume is almost always different than the solution volume.
Ilkovic equation → Ilkovičeva jednadžba
Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form
Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3.
The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980).
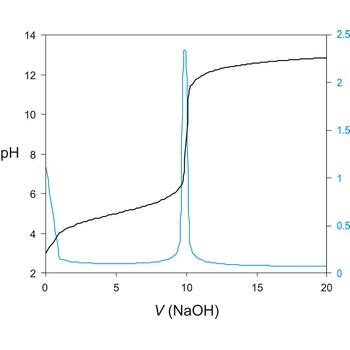
potentiometric titration → potenciometrijska titracija
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
saturated solution → zasićena otopina
Saturated solution is a solution that holds the maximum possible amount of dissolved material. When saturated, the rate of dissolving solid and that of recrystallisation solid are the same, and a condition of equilibrium is reached. The amount of material in solution varies with temperature; cold solutions can hold less dissolved solid material than hot solutions. Gases are more soluble in cold liquids than in hot liquids.
absorbance → apsorbancija
Absorbance (A) is a logarithm of the ratio of incident radiant power (Po) to transmitted radiant power (P) through a sample (excluding the effects on cell walls).
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
Citing this page:
Generalic, Eni. "Maksimalno dopustiva koncentracija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table