logarithmic scale → logaritamska skala
Logarithmic scale is the one in which values of 1, 2, 3, 4, 5, in fact represents values of 1, 10, 100, 1 000, 10 000. Logarithmic scales are often used to simplify graphs and tables, where otherwise changes of data at the lower end of the scale would be difficult to distinguish (e.g. a graph axis which would normally have values from 1 - 1 000 000 is shown by values of 1 - 7). An example of a logarithmic scale is the pH scale.
mineral → mineral
Minerals are compounds in which metals can be found in nature. Metals in nature can appear as:
| autochthonous | Au, Cu, Pt, Ag, Pd, Hg, Ir |
| oxides | Fe, Al, Sn, Cr, Mn, W, Cu |
| sulphides | Cu, Pb, Zn, Ni, Ag, Co, Sb, Hg, Mo, Cd, Bi |
| carbonates | Fe, Zn, Cu, Mg, Mn, Pb |
| silicates | Ni, Cu, Zn, Mn |
| chlorides | Ag, Cu, Mg, Na, K |
| sulphates | Ca, Ba, Sr, Cu |
Moh’s scale → Mohsova skala
Mohs’ scale of mineral hardness characterises the scratch resistance of various minerals through the ability of a harder material to scratch a softer. It was created by the German mineralogist Friedrich Mohs (1773-1839). Mohs based the scale on the ten readily available minerals.
| Hardness | Mineral |
|---|---|
| 1 | talc (Mg3Si4O10(OH)2) |
| 2 | gypsum (CaSO4·2H2O) |
| 3 | calcite (CaCO3) |
| 4 | fluorite (CaF2) |
| 5 | apatite (Ca5(PO4)3(OH-,Cl-,F-)) |
| 6 | orthoclase feldspar (KAlSi3O8) |
| 7 | quartz (SiO2) |
| 8 | topaz (Al2SiO4(OH-,F-)2) |
| 9 | corundum (Al2O2) |
| 10 | diamond (C) |
nerve poison → živčani bojni otrov
Nerve poison (nerve gas, agents) have had an entirely dominant role since the Second World War. Nerve poisons acquired their name because they affect the transmission of nerve impulses in the nervous system. All nerve poisons belong chemically to the group of organo-phosphorus compounds. They are stable and easily dispersed, highly toxic and have rapid effects both when absorbed through the skin and via respiration. Nerve poisons can be manufactured by means of fairly simple chemical techniques. The raw materials are inexpensive and generally readily available.
The most important nerve agents included in modern chemical weapons arsenals are:
| Tabun | (o-ethyl dimethylamidophosphorylcyanide) |
| Sarin | (isopropyl methylphosphonofluoridate) |
| Soman | (pinacolyl methylphosphonofluoridate) |
| GF | (cyclohexyl methylphosphonofluoridate) |
| VX | (o-ethyl S-diisopropylaminomethyl methylphosphonothiolate) |
Nerve poisons are colorless, odorless, tasteless liquids of low volatility. Antidotes are atropine sulfate and pralidoxime iodide.
referent electrode → referentna elektroda
Referent electrode is an electrode whose potential is known and completely independent of analyte concentration. Mostly used referent electrodes are calomel and silver/silver chloride electrode.
Table: Dependence of referent electrodes potentials on KCl concentration
| Potential vs. SHE / V | |||||
| calomel electrode | Ag/AgCl electrode | ||||
| t / °C | 0.1 mol dm-3 | 3.5 mol dm-3 | sat. solution | 3.5 mol dm-3 | sat. solution |
| 15 | 0.3362 | 0.254 | 0.2511 | 0.212 | 0.209 |
| 20 | 0.3359 | 0.252 | 0.2479 | 0.208 | 0.204 |
| 25 | 0.3356 | 0.250 | 0.2444 | 0.205 | 0.199 |
| 30 | 0.3351 | 0.248 | 0.2411 | 0.201 | 0.194 |
| 35 | 0.3344 | 0.246 | 0.2376 | 0.197 | 0.189 |
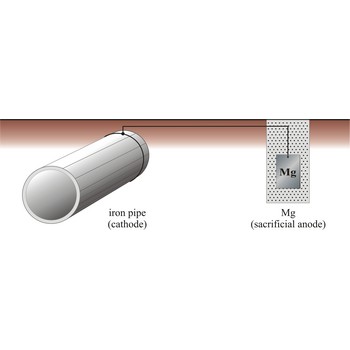
sacrificial protection → zaštita žrtvovanom elektrodom
Sacrificial protection is the protection of iron or steel against corrosion by using a more reactive metal. Pieces of zinc or magnesium alloy are attached to pump bodies and pipes. The protected metal becomes the cathode and does not corrode. The anode corrodes, thereby providing the desired sacrificial protection. These items are known as sacrificial anodes and "attract" the corrosion to them rather than the iron/steel. The sacrificial anodes must be replaced periodically as they corrode.
The iron pipe will be connected to a more reactive metal such as magnesium through cooper wires, the magnesium will donate its electrons to the iron preventing it from rusting. Iron which is oxidises will immediately be reduced back to iron.
salt water → slana voda
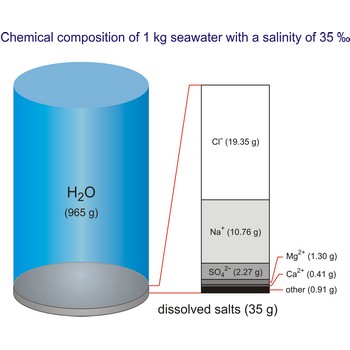
Salt water is the water of the sea and the ocean. This water contains a relatively high percentage of dissolved salt (about 35 g of salt per 1 000 g of sea water.). About 90 % of that salt would be sodium chloride, or ordinary table salt.
The salinity of ocean water varies. It is affected by such factors as melting of ice, inflow of river water, evaporation, rain, etc.
saturated fatty acid → zasićena masna kiselina
Saturated fatty acid is a fatty acid carrying the maximum possible number of hydrogen atoms (It doesn’t have any double bounds in the alkyl chain). The most important of these are:
| Butyric (butanoic acid) | CH3(CH2)2COOH |
| Lauric (dodecanoic acid) | CH3(CH2)10COOH |
| Myristic (tetradecanoic acid) | CH3(CH2)12COOH |
| Palmitic (hexadecanoic acid) | CH3(CH2)14COOH |
| Stearic (octadecanoic acid) | CH3(CH2)16COOH |
| Arachidic (eicosanoic acid) | CH3(CH2)18COOH |
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
significant figures → značajne znamenke
Measurements are not infinitely accurate: we must estimate measurement uncertainty. The number of significant figures is all of the certain digits plus the first uncertain digit.
Rules for significant figures:
- Disregard all initial zeros.
- Disregard all final zeros unless they follow a decimal point.
- All remaining digits including zeros between nonzero digits are significant.
| 0.0023 | has two significant figures |
| 0.109 | has three significant figures |
| 2.00 | has three significant figures |
| 70 | has one significant figure |
In addition and subtraction, the number of significant figures in the answer depends on the original number in the calculation that has the fewest digits to the right of the decimal point.
In multiplication and division, the number of significant figures in a calculated result is determined by the original measurement that has the fewest number of significant digits.
In a logarithm of a number, keep as many digits to the right of the decimal point as there are significant figures in the original number.
In an antilogarithm of a number, keep as many digits as there are digits to the right of the decimal point in the original number.
Citing this page:
Generalic, Eni. "La primer tabla periodica." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table