filter paper → filtar papir
Filter paper is a quantitative paper used for filtering and made of pure cellulose treated with hydrochloric and hydrofluoric acid. This kind of paper burns out practically without any remains (less than 0.0001 g ashes). Different types of paper are marked with numbers; qualitative bears marking 595 or 597 and quantitative 589 or 590. Dependable upon precipitate character, different types of filter paper are used:
- black band (5891) - 100 mL of fluid flows through it in 20 s to 30 s. It is used for filtering of gelatinous precipitates.
- white band (5892) - 100 mL of fluid flows through it in 40 s to 60 s. It is used for coarse crystalline precipitates filtration.
- blue band (5893) - 100 mL of fluid flows through it in 200 s to 400 s. It is used for fine crystalline precipitates.
Lyman series → Lymanova serija
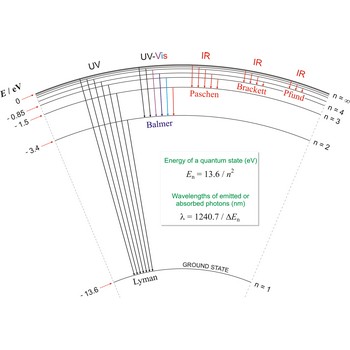
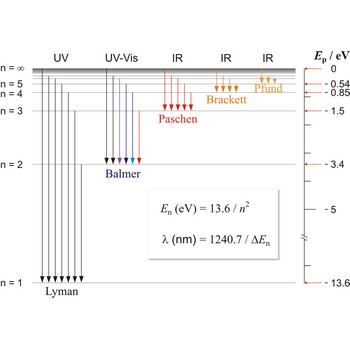
Lyman series is the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the ground state (principal quantum number n = 1) and successive excited states.
Pauli exclusion principle → Paulijev princip zabrane
Pauli exclusion principle is the statement that two electrons in an atom cannot have identical all four quantum numbers. It was first formulated in 1925 by the Austrian-born Swiss physicst Wolfgang Ernst Pauli (1900-1958).
rate equation → jednadžba brzine reakcije
Rate equation is an equation that describes the dependence of reaction rate on concentrations of reacting species. It always has the form
where a and b are usually integral exponents.
orbital → orbitala
Orbital is the area in space about an atom or molecule in which the probability of finding an electron is greatest.
The possible atomic orbitals correspond to subshells of the atom. Thus there is one s-orbital for each shell (orbital quantum number l = 0). There are three p-orbitals (corresponding to the three values of l) and five d-orbitals. The shapes of orbitals depend on the value of l.
Paschen series → Paschenova serija
Paschen series are the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the state with principal quantum number n = 3 and successive higher states.
spin → spin
Spin is the intrinsic angular momentum of an elementary particle, or system of particles such as nucleus, that is also responsible for the magnetic moment; or, a particle or nucleus possessing such a spin. The spins of nuclei have characteristic fixed values. Pairs of neutrons and protons align to cancel out their spins, so that nuclei with an odd number of neutrons and/or protons will have a net non-zero rotational component characterized by a non-zero quantum nuclear spin number.
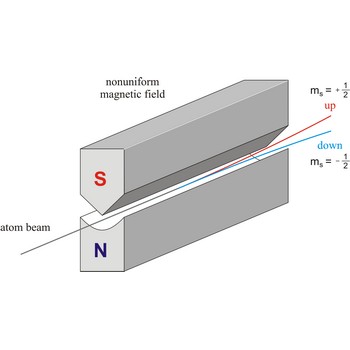
Stern-Gerlach experiment: a beam of silver atoms is split into two beams when it traverses a nonuniform magnetic field. Atoms with spin quantum number ms=+1/2 follow one trajectory, and those with ms=+1/2 follow another.
transference number → prijenosni broj
Transference number of an ion is the fraction of the total current that is carried by that ion during electrolysis. Different ions carry different fractions of the current because different ions move at different speeds under the same potential gradient. In general, a cation and an anion differ in the amount of current they can carry during electrolysis.
valence shell → valentna ljuska
Valence shell is the shell corresponding to the highest value of principal quantum number in the atom. The valence electrons in this shell are on average farther from the nucleus than other electrons. They are often directly involved in chemical reaction.
Citing this page:
Generalic, Eni. "Kvantni brojevi." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table