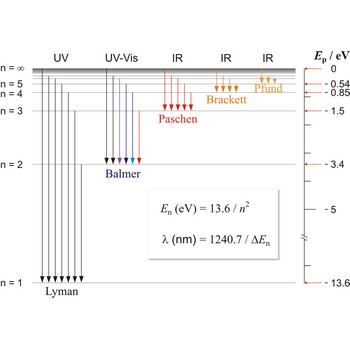
Paschen series → Paschenova serija
Paschen series are the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the state with principal quantum number n = 3 and successive higher states.
photoelectric effect → fotoelektrični efekt
Photoelectric effect is the complete absorption of a photon by a solid with the emission of an electron. The energy of a photon (hν) is
Planck constant → Planckova konstanta
Planck constant (h) is a constant that, when multipled by the frequency of radiation gives the quantity of energy contained in one quantum.
Equal to 6.626 075 5(40)·10-34 J s. It was named after Max Planck (1858-1947).
solution composition → sastav otopine
Solutions are homogenous mixtures of several components. The component which is found in a greater quantity is called the solvent and the other components are called solutes. Quantitative composition of a solution can be expressed by concentration (amount, mass, volume and number), by fraction (amount, mass, and volume), ratio (amount, mass, and volume) and by molality. Amount, mass, and volume ratio are numerical, nondimensional units and are frequently expressed as percentage (% = 1/100), promile (‰ = 1/1000) or parts per million (ppm = 1/1 000 000). If it is not defined, it is always related to the mass ratio.
spin → spin
Spin is the intrinsic angular momentum of an elementary particle, or system of particles such as nucleus, that is also responsible for the magnetic moment; or, a particle or nucleus possessing such a spin. The spins of nuclei have characteristic fixed values. Pairs of neutrons and protons align to cancel out their spins, so that nuclei with an odd number of neutrons and/or protons will have a net non-zero rotational component characterized by a non-zero quantum nuclear spin number.
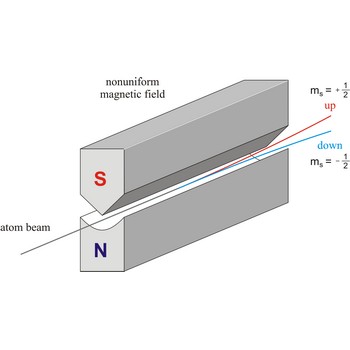
Stern-Gerlach experiment: a beam of silver atoms is split into two beams when it traverses a nonuniform magnetic field. Atoms with spin quantum number ms=+1/2 follow one trajectory, and those with ms=+1/2 follow another.
stoichiometry → stehiometrija
Stoichiometry is the relative proportions elements from compounds or in which substances react. Every chemical reaction has its characteristic proportions. For example, when methane unites with oxygen in complete combustion, 1 mol of methane requires 2 mol of oxygen.
At the same time, 1 mol of carbon dioxide and 2 mol of water are formed as reaction products.
Alternatively, 16 g of methane and 64 g of oxygen produce 44 g of carbon dioxide and 36 g of water.
The stoichiometric relationship between the products and reactants can be used to in calculations.
titrant → titrant
Titrant is the substance that quantitatively reacts with the analyte in a titration. The titrant is usually a standard solution added carefully to the analyte until the reaction is complete. The amount of analyte is calculated from the volume and concentration of titrant required for the complete reaction.
valence shell → valentna ljuska
Valence shell is the shell corresponding to the highest value of principal quantum number in the atom. The valence electrons in this shell are on average farther from the nucleus than other electrons. They are often directly involved in chemical reaction.
Citing this page:
Generalic, Eni. "Kvant." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table