chromium → krom
Chromium was discovered by Louis-Nicholas Vauquelin (France) in 1797. The origin of the name comes from the Greek word chroma meaning colour. It is very hard, crystalline, steel-grey metal. The pure metal has a blue-white colour. It is hard, brittle and corrosion-resistant at normal temperatures. Hexavalent compounds toxic by skin contact. The most important chromium mineral is chromite [Fe,Mg(CrO4)]. Produced commercially by heating its ore in the presence of silicon or aluminium. Used to make stainless steel. It gives the colour to rubies and emeralds. Iron-nickel-chromium alloys in various percentages yield an incredible variety of the most important metals in modern technology.
chromatography → kromatografija
Chromatography is a method of separation of the components of a sample in which the components are distributed between two phases, one of which is stationary while the other moves. In gas chromatography, the gas moves over a liquid or solid stationary phase. In liquid chromatography, the liquid mixture moves through another liquid, a solid, or a gel. The mechanism of separation of components may be adsorption, differential solubility, ion-exchange, permeation, or other mechanisms.
column chromatography → kromatografija u koloni
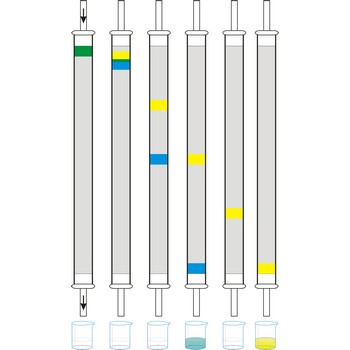
Column chromatography is generally used as a purification technique: it isolates desired compounds from a mixture. In column chromatography, the stationary phase, a solid adsorbent, is placed in a vertical column. The mobile phase, a liquid, is added to the top and flows down through the column by either gravity or external pressure. The mobile phase can be a gas or a liquid which gives rise to the two basic forms of chromatography, namely, gas chromatography (GC) and liquid chromatography (LC).
paper chromatography → papirna kromatografija
Paper chromatography is one of the types of chromatography procedures which runs on a piece of specialized paper. It is a planar chromatography systems wherein a cellulose filter paper acts as a stationary phase on which separation of compounds occurs. The edge of the paper is immersed in a solvent, and the solvent moves up the paper by capillary action.
supercritical fluid chromatography → superkritična fluidna kromatografija
Supercritical fluid chromatography (SFC) is a hybrid of gas and liquid chromatography. SFC is of importance because it permits the separation and determination of a group of compounds that are not conveniently handled by either gas or liquid chromatography. These compounds are either nonvolatile or thermally labile so that gas chromatography cannot be used and they do not contain functional groups that make possible detection by liquid chromatography. SFC has been applied to a wide variety of materials including natural prodcuts, drugs, foods, pesticides and herbicides, fossil fuels, explosives and propellants.
thin layer chromatography → tankoslojna kromatografija
Thin layer chromatography. (TLC) is a technique for separating components in a mixture on the basis of their differing polarities. A spot of sample is placed on a flat sheet coated with silica and then carried along by a solvent that soaks the sheet. Different components will move different distances over the surface. TLC is a useful screening technique in clinical chemistry; for example, it can be used to detect the presence of drugs in urine.
absorbance → apsorbancija
Absorbance (A) is a logarithm of the ratio of incident radiant power (Po) to transmitted radiant power (P) through a sample (excluding the effects on cell walls).
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
absorption coefficient → apsorpcijski koeficijent
Absorption coefficient (a) is the relative decrease in the intensity of a collimated beam of electromagnetic radiation, as a result of absorption by a medium, during traversal of an infinitesimal layer of the medium, divided by the length traversed.
Allihn condenser → Allihnovo hladilo
Allihn condenser or bulb condenser consists of an outer water jacket and the inner glass tube with a series of spherical bubbles to maximize the thermal contact with the cooling water. It is named after its inventor, the German chemist Felix Richard Allihn (1854-1915).
amperometry → amperometrija
Amperometry is determining the concentration of a material in a sample by measuring electric current passing through a cell containing the solution.
Citing this page:
Generalic, Eni. "Krom." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table