hypsometric curve → hipsometrijska krivulja
Hypsometric curve (or hypsographic curve) shows the distribution of height of a given area (on land) and depth (at sea). The term originates from the Greek word hypsos meaning height. The part of the curve that reflects the cross section of the ocean bottom is called the bathygraphic curve.
Horizontal dashed lines indicate average height of the continents at 840 meters above sea level, and average depth of the oceans at 3 682.2 meters below sea level. If all the land above sea level (green) was moved into the sea (blue), the oceans would still be 3 km deep.
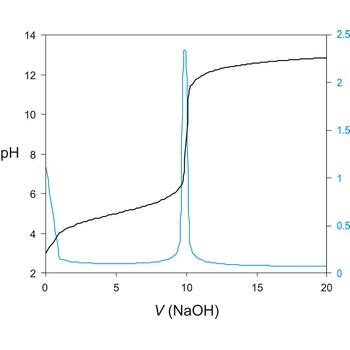
titration curve → titracijska krivulja
Titration curve is a graphic representation of the amount of a species present vs. volume of solution added during a titration. A titration curve has a characteristic sigmoid curve. The inflection point in the titration curve marks the end-point of the titration. Blue line is the first derivative of the titration curve.
acylaction reaction → reakcije aciliranja
Acylaction reaction involves the introduction of an acyl group (RCO-) into a compound. An alkyl halide is reacted with an alcohol or a carboxylic acid anhydride e.g.
The introduction of an acetyl group (CH3CO-) is acetylation, a process used for protecting -OH groups in organic synthesis.
chain reaction → lančana reakcija
Chain reaction is a reaction done in three steps: initiation in which usually radicals are made which react with other molecules in stage of propagation and, when all reactants are spent, it ends by termination when all available radicals are completely spent.
Nuclear chain reaction refers to a process in which neutrons released in fission produce an additional fission in at least one further nucleus. Nuclear power plants operate by precisely controlling the rate at which nuclear reactions occur. On the other hand, nuclear weapons are specifically engineered to produce a reaction that is so fast and intense it cannot be controlled after it has started.
zero-order reaction → reakcija nultog reda
Zero-order reaction is a reaction for which the rate of reaction is independent of the concentration of reactants.
reversible reaction → reverzibilna reakcija
Reversible reaction is a chemical reaction that can proceed in both the forward and backward directions. When reversible reactions reach equilibrium the forward and reverse reactions are still happening but at the same rate, so the concentrations of reactants and products do not change. A reversible reaction is denoted by a double arrow pointing both directions in a chemical equation.
velocity → brzina
If a point-like object moves so that its position vector changes from being ri to rf, than the displacement Δr of object is
If a point-like object undergoes a displacement, Δr, in time Δt, its average velocity, v is defined as
The instantaneous velocity, v, is obtained from the average velocity by shrinking the time interval Δt towards zero. The average velocity approaches a limiting value, which is the velocity of a given instant:
Velocity is a vector quantity. If we plot the path of a moving particle as a curve in a coordinate system, the instantaneous velocity is always tangent to that curve.
SI unit for velocity is m s-1.
chemical reaction → kemijska reakcija
Chemical reaction is a change of chemical properties of substances which react with each other. By means of a chemical reaction new substances are created by bond breaking between atoms and molecules of reactants and their reuniting in a new way, thereby creating products. Chemical reactions can be shown by chemical equations.
angular velocity → kutna brzina
A point-like object that undergoes circular motion changes its angular position from initial Θi to final Θf, relative to a fixed axis, specified in a coordinate system with an origin that coincides the centre of the circular path of object. The change in its angular position is called angular displacement ΔΘ = Θf - Θi. Also, a rigid body that rotates about a specified rotation axis, changing its angular position from initial Θi to final Θf, undergoes an angular displacement ΔΘ.
The average angular velocity, ωav, is the ratio of the angular displacement and the time interval Δt=tf-ti, in which that displacement occurs.
Θf and Θi are the initial and final angular position, respectively.
The instantaneous angular velocity ω is the limit of the average angular velocity, as Δt is made to approach zero.
ωav and ω are positive for the counterclockwise rotation (in direction of increasing Θ) and negative for the clockwise rotation (in direction of decreasing Θ).
SI unit for angular velocity is s-1.The measure for the angle Θ is radian. The relationship between radians and degrees is:
For example, the angular velocity of the minute hand of a clock is:
elementary reaction → elementarna reakcija
Elementary reaction is a reaction that occurs in a single step. Equations for elementary reactions show the actual molecules, atoms, and ions that react on a molecular level.
Citing this page:
Generalic, Eni. "Krivulja brzine reakcije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table