alkaline earth metal → zemnoalkalijski metal
Alkali earth metal is a term that refers to six elements: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). These elements make up group 2 of the periodic table of elements. They all exhibit a single oxidation state, +2. They are all light and very reactive. Barium and radium are the most reactive and beryllium is the least.
To denote slightly soluble metal oxides chemists formerly used the term "earth". The oxides of barium, strontium, and calcium resemble alumina (Al2O3), a typical "earth", but form alkaline mixtures with water. For this reason barium, strontium, and calcium were called alkaline earth metals. This name has now been extended to include all of the elements of group 2.
berkelium → berkelij
Berkelium was discovered by Stanley G. Thompson, Albert Ghiorso and Glenn T. Seaborg (USA) in 1949. Named after Berkeley, a city in California, home of the University of California, USA. It is synthetic radioactive metal. Berkelium was made by bombarding americium with alpha particles.
Bravais lattice → Bravaisova rešetka
Bravais lattice is a set of points constructed by translating a single point in discrete steps by a set of basis vectors. The French crystallographer Auguste Bravais (1811-1863) established that in three-dimensional space only fourteen different lattices may be constructed. All crystalline materials recognised till now fit in one of these arrangements. The fourteen three-dimensional lattices, classified by crystal system, are shown to the bottom.
|
Crystal system
|
Bravais lattices
|
|||
|
cubic a=b=c α=β=γ=90° |
 |
 |
 |
|
|
|
simple cubic
|
body-centered cubic
|
face-centered cubic
|
|
|
tetragonal a=b≠c α=β=γ=90° |
 |
 |
||
|
|
simple tetragonal
|
body-centered tetragonal
|
||
|
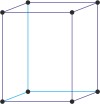
orthorhombic a≠b≠c α=β=γ=90° |
 |
 |
 |
 |
|
|
simple orthorhombic
|
base-centered orthorhombic
|
body-centered orthorhombic
|
face-centered orthorhombic
|
|
monoclinic a≠b≠c α=γ=90°≠β |
 |
 |
||
|
|
simple monoclinic
|
base-centered monoclinic
|
||
|
hexagonal a=b≠c α=β=90° γ=120° |
 |
|||
|
|
hexagonal
|
|||
|
rhombohedral a=b=c α=β=γ≠90° |
 |
|||
|
|
rhombohedral
|
|||
|
triclinic a≠b≠c α≠β≠γ≠90° |
 |
|||
|
triclinic
|
||||
Allihn condenser → Allihnovo hladilo
Allihn condenser or bulb condenser consists of an outer water jacket and the inner glass tube with a series of spherical bubbles to maximize the thermal contact with the cooling water. It is named after its inventor, the German chemist Felix Richard Allihn (1854-1915).
californium → kalifornij
Californium was discovered by Stanley G. Thompson, Kenneth Street Jr. and Albert Ghiorso (USA) in 1950. Named after the State and University of California, USA. It is synthetic radioactive metal. Powerful neutron emitter. Californium was made by bombarding curium with helium ions.
aluminium → aluminij
Aluminium was discovered by Friedrich Wöhler (Germany) in 1827. The origin of the name comes from the Latin word alumen meaning alum. It is soft, lightweight, silvery-white metal. Exposed surfaces quickly form protective oxide coating. Metal reacts violently with oxidants. Third most abundant element in the earth’s crust. Aluminium is the most abundant metal to be found in the earth’s crust, but is never found free in nature. Aluminium is obtained by electrolysis from bauxite. Used for many purposes from airplanes to beverage cans. Too soft in its pure form so less than 1 % of silicon or iron is added, which hardens and strengthens it.
ampere → amper
Ampere (A) is the SI base unit of electric current.
The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 metre apart in vacuum, would produce between these conductors a force equal to 2×10-7 newton per metre of length.
anhydrous → anhidrid
Anhydrous (without water) is an applied to minerals which do not contain water of crystallization or water of chemical combination. For example, strongly heated copper (II) sulphate pent hydrate (CuSO4•5H2O) produces anhydrous copper (II) sulphate (CuSO4). Less stable and more dangerous to use than hydrated.
aqua regia → zlatotopka
Aqua regia is a mixture of one volume part of nitric acid (HNO3) and three volume parts of hydrochloric acid (HCl). Dissolving of gold in aqua regia is described by the following equation:
Citing this page:
Generalic, Eni. "Kristalna voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table


