Faraday’s laws of electrolysis → Faradayevi zakoni elektrolize
Faraday’s laws of electrolysis are two laws found by British chemist and physicist Michael Faraday (1791-1867) in his experiments on electrolysis:
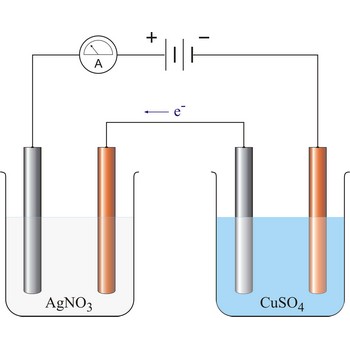
1. The quantity of matter extracted on the electrode is proportional to the quantity of charge (Q = I·t) which has flown in electrolysis time.
where z = number of electrons changed in reaction and F = Faraday’s constant which equals 96 487 C mol-1.
2. The masses of the elements liberated by the same quantity of electricity are directly proportional to their chemical equivalents.
96 487 C will discharge 1 mol Ag and 1/2 mol Cu. The relevant half reactions are:
ferromagnetism → feromagnetizam
Ferromagnetism is a type of magnetism in which the magnetic moments of atoms in a solid are aligned within domains which can in turn be aligned with each other by a weak magnetic field. The total magnetic moment of a sample of the substance is the vector sum of the magnetic moments of the component domains. In an unmagnetized piece of ferromagnetic material the magnetic moments of the domains themselves are not aligned; when an external field is applied those domains that are aligned with the field increase in size at the expense of the others. Ferromagnetic materials can retain their magnetisation when the external field is removed, as long as the temperature is below a critical value, the Curie temperature. They are characterised by a large positive magnetic susceptibility.
glass → staklo
Glass is a brittle transparent solid with the molecular structure of a liquid. It is made by fusing together sand (SiO2), soda (Na2CO3), and lime (CaCO3) with small quantities other compounds. It is used for window panes and mirrors, for articles of table and culinary use, for lenses, and various articles of ornament.
Glauber’s salt → Glauberova sol
Glauber’s salt is sodium sulphate decahydrate (Na2SO4·10H2O). Loses water of hydration at 100 °C. Energy storage capacity is more than seven times that of water.
Heat of atomisation → toplina atomiziranja
Heat of atomisation or enthalpy of atomisation is the energy required to dissociate one mole of a given substance into atoms.
heat of combustion → toplina izgaranja
Heat of combustion or enthalpy of combustion is the heat evolved when a definite quantity of a substance is completely oxidised (burned).
heat of crystallization → toplina kristalizacije
Heat of crystallization or enthalpy of crystallization is the heat evolved or absorbed when one mole of given substance crystallises from a saturated solution of the same substance.
filtration → filtriranje
Filtration is a procedure in which liquids are separated from the precipitate by passing a suspension through the filter. The precipitate remains on the filter and through it the filtrate passes. Gaseous heterogeneous mixtures can also be filtrated.
foam → pjena
Foams are dispersions of gases in liquids or solids. The gas globule may be of any size, from colloidal to macroscopic, as in soap bubbles. Bakers’ bread and sponge rubber are examples of solid foams. Typical liquid foams are those used in fire-fighting, shaving creams, etc. Foams made by mechanical incorporation of air are widely used in the food industry (e.g. whipped cream, egg white, ice cream, etc.). Foams can be stabilized by surfactants.
fuel cell → gorivi članak
Fuel cell is a device that converts chemical energy into electrical energy. It is different from a battery in that the energy conversion continues as long as fuel and oxidising agent are fed to the fuel cell; that is, in principle indefinitely. (A battery is manufactured with a limited amount of chemicals, and it is exhausted when all the chemicals have reacted.) It is a galvanic cell where spontaneous chemical reactions occur at the electrodes. The fuel is oxidised at the anode, and the oxidising agent (almost always oxygen or air) is reduced at the cathode. Presently, the most commonly used fuel is hydrogen. More conventional fuels (e.g., petrol or natural gas) must be converted (reformed) into hydrogen before they can be utilised in a fuel cell.
Some fuel cells employ an aqueous solution as electrolyte, that can be either acidic or basic (alkaline), or an ion-exchange membrane soaked in aqueous solution can act as the electrolyte. These fuel cells operate at relatively low temperatures (from room temperature to not much above the boiling point of water). Some fuel cells employ molten salts (especially carbonates) as electrolytes and have to operate at temperatures of several hundred degrees centigrade (Celsius). Others employ ionically conductive solids as electrolyte and must operate close to 1 000 °C.
Citing this page:
Generalic, Eni. "Kristalna tvar." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table