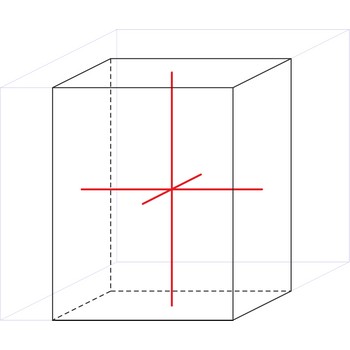
crystal system → kristalni sustav
Crystal system is a method of classifying crystalline substances on the basis of their unit cell. There are seven unique crystal systems. The simplest and most symmetric, the cubic (or isometric) system, has the symmetry of a cube. The other six systems, in order of decreasing symmetry, are hexagonal, tetragonal, rhombohedral (also known as trigonal), orthorhombic, monoclinic and triclinic.
|
Crystal system
|
Unit-cell
|
Conditions on unit-cell edges and angles |
|
cubic |
 |
a=b=c α=β=γ=90° |
|
hexagonal |
 |
a≠c α=γ=90° β=120° |
|
tetragonal |
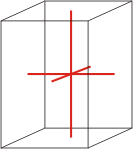 |
a=b≠c α=β=γ=90° |
|
rhombohedral |
 |
a=b=c α=β=γ≠90° |
|
orthorhombic |
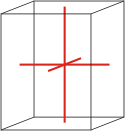 |
a≠b≠c α=β=γ=90° |
|
monoclinic |
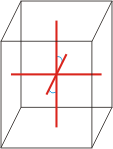 |
a≠b≠c α=γ=90°≠β |
|
triclinic |
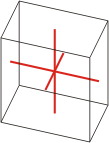 |
a≠b≠c α≠β≠γ≠90° |
cubic crystal system → kubični kristalni sustav
Cubic crystal system is also known as the isometric system. The Isometric crystal system characterizes itself by its three equivalent crystallographic axes perpendicular to each other.
a = b = c
α = β = γ = 90°
dielectric constant → dielektrična konstanta
Dielectric constant or permittivity (ε) is an index of the ability of a substance to attenuate the transmission of an electrostatic force from one charged body to another. The lower the value, the greater the attenuation. The standard measurement apparatus utilises a vacuum whose dielectric constant is 1. In reference to this, various materials interposed between the charged terminal have the following value at 20 °C:
| vacuum | 1 |
| air | 1.00058 |
| glass | 3 |
| benzene | 2.3 |
| acetic acid | 6.2 |
| ammonia | 15.5 |
| ethanol | 25 |
| glycerol | 56 |
| water | 81 |
The exceptionally high value for water accounts for its unique behaviour as a solvent and in electrolytic solutions. Dielectric constant values decrease as the temperature rises.
Planck constant → Planckova konstanta
Planck constant (h) is a constant that, when multipled by the frequency of radiation gives the quantity of energy contained in one quantum.
Equal to 6.626 075 5(40)·10-34 J s. It was named after Max Planck (1858-1947).
dissociation constant → konstanta disocijacije
Dissociation constant is a constant whose numerical value depends on the equilibrium between the undissociated and dissociated forms of a molecule. A higher value indicates greater dissociation.
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
The equilibrium constant of such a dissociation is called the acid dissociation constant or acidity constant, given by
The concentration of water [H2O] can be taken as constant.
Similarly, for a base, the equilibrium
is also a dissociation; with the base dissociation constant or basicity constant, given by
Ka (Kb) is a measure of the strength of the acid (base).
universal gas constant → univerzalna plinska konstanta
Universal gas constant R has the value of 8.314 472(15) J K-1 mol-1. It corresponds to the volume work performed by one mole of gas heated by 1 K at standard pressure.
equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
hexagonal crystal system → heksagonski kristalni sustav
Hexagonal crystal system is based on four crystallographic axes. The system of crystallographic axes of the hexagonal crystal system consists of three equivalent horizontal (equatorial) axes of which the positive ends make an angle of 120°. These axes are sometimes denoted as a, b and d axes. The fourth axis is (c) is perpendicular to and shorter or longer than the other three.
monoclinic crystal system → monoklinski kristalni sustav
Minerals of the monoclinic crystal system are referred to three unequal axes. Two of these axes (a and c) are inclined toward each other at an oblique angle; these are usually depicted vertically. The third axis (b) is perpendicular to the other two and is called the ortho axis. The two vertical axes therefore do not intersect one another at right angles, although both are perpendicular to the horizontal axis.
a ≠ b ≠ c
α = γ = 90° ≠ β
orthorhombic crystal system → ortorompski kristalni sustav
Orthorhombic crystal system is also known as the rhombic system. Minerals of the orthorhombic crystal system are referred to three mutually perpendicular axes, each of which is of a different length than the others.
a ≠ b ≠ c
α = β = γ = 90°
Citing this page:
Generalic, Eni. "Konstante kristalne rešetke." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table