water ion product → ionski produkt vode
Water ion product (Kw) is a concentration product of hydrogen and hydroxide ions. For the reaction:
the equilibrium expression would be:
Note that all pure liquid terms are omitted, hence H2O does not appear in the denominator. At 25 °C, Kw = 1.0×10-14 mol2dm-6 = (Ka)(Kb)
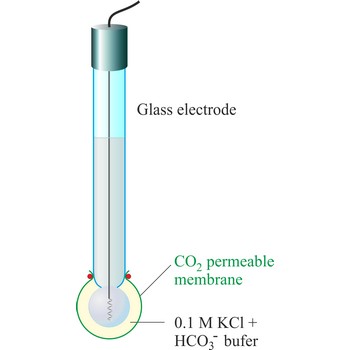
CO2 ion selective electrode → CO2 ion selektivna elektroda
The carbon dioxide ion selective electrode uses a gas-permeable membrane to separate the sample solution from the electrode internal solution. Dissolved carbon dioxide in the sample solution diffuses through the membrane until an equilibrium is reached between the partial pressure of CO2 in the sample solution and the CO2 in the internal filling solution. In any given sample the partial pressure of carbon dioxide will be proportional to the concentration of carbon dioxide. The diffusion across the membrane affects the level of hydrogen ions in the internal filling solution:
The hydrogen level of the internal filling solution is measured by the pH electrode located behind the membrane. The internal filling solution contains a high concentration of sodium bicarbonate (e.g. 0.1 mol/L NaHCO3) so that the bicarbonate level can be considered constant.
acid → kiselina
Acid is a type of compound that contains hydrogen and dissociates in water to produce positive hydrogen ions. The reaction for an acid HA is commonly written:
In fact, the hydrogen ion (the proton) is solvated, and the complete reaction is:
This definition of acids comes from the Arrhenius theory. Such acids tend to be corrosive substances with a sharp taste, which turn litmus red and produce colour changes with other indicators. They are referred to as protonic acids and are classified into strong acids, which are almost completely dissociated in water, (e.g. sulphuric acid and hydrochloric acid), and weak acids, which are only partially dissociated (e.g. acetic acid and hydrogen sulphide). The strength of an acid depends on the extent to which it dissociates, and is measured by its dissociation constant.
In the Lowry-Brønsted theory of acids and bases (1923), the definition was extended to one in which an acid is a proton donor (a Brønsted acid), and a base is a proton acceptor (a Brønsted base). An important feature of the Lowry-Brønsted concept is that when an acid gives up a proton, a conjugate base is formed that is capable of accepting a proton.
Similarly, every base produces its conjugate acid as a result of accepting a proton.
For example, acetate ion is the conjugate base of acetic acid, and ammonium ion is the conjugate acid of ammonia.
As the acid of a conjugate acid/base pair becomes weaker, its conjugate base becomes stronger and vice versa.
A further extension of the idea of acids and bases was made in the Lewis theory. In this, a G. N. Lewis acid is a compound or atom that can accept a pair of electrons and a Lewis base is one that can donate an electron pair. This definition encompasses "traditional" acid-base reactions, but it also includes reactions that do not involve ions, e.g.
in which NH3 is the base (donor) and BCl3 the acid (acceptor).
air curtain → zračna zavjesa
Air curtain is a constant stream of bubbles provided by a submerged diffuser (usually a tube type), which surrounds a specified area.
amperometry → amperometrija
Amperometry is determining the concentration of a material in a sample by measuring electric current passing through a cell containing the solution.
azeotrope → azeotropi
Azeotrope is a mixture of two liquids that boils at constant composition, i.e. the composition of the vapour is the same as that of the liquid. Azeotropes occur because of deviations in Raoult’s law leading to a maximum or minimum in the boiling point - composition diagram. The composition of an azeotrope depends on the pressure.
analytical balance → analitička vaga
Analytical balances are instruments used for precise determining mass of matter. Analytical balances are sensitive and expensive instruments, and upon their accuracy and precision the accuracy of analysis result depends. The most widely used type of analytical balances are balances with a capacity of 100 g and a sensitivity of 0.1 mg. Not one quantitative chemical analysis is possible without usage of balances, because, regardless of which analytical method is being used, there is always a need for weighing a sample for analysis and the necessary quantity of reagents for solution preparation.
The working part of the balance is enclosed in a glass-fitted case. The baseplate is usually of black glass or black slate. The beam has agate knife-edges at its extremes, supporting stirrups from which balance pans are suspended. Another agate or steel knife-edge is fixed exactly in the middle of the beam on its bottom side. This knife-edge faces downwards and supports the beam. When not in use and during loading or unloading of the pans, the balance should be arrested.
The principle of operation of a modern laboratory balance bears some resemblance to its predecessor - the equal arm balance. The older instrument opposed the torque exerted by an unknown mass on one side of a pivot to that of an adjustable known weight on the other side. When the pointer returned to the center position, the torques must be equal, and the weight was determined by the position of the moving weights.
Modern electronic laboratory balances work on the principle of magnetic force restoration. In this system, the force exerted by the object being weighed is lifted by an electromagnet. A detector measures the current required to oppose the downward motion of the weight in the magnetic field.
Boltzmann equation → Boltzmannova jednadžba
Boltzmann equation is a statistical definition of entropy, given by
where S and k are the entropy and Boltzmann’s constant, respectively, and W is the probability of finding the system in a particular state.
arginine → arginin
Arginine is an electrically charged amino acids with basic side chains. It is one of the least frequent amino acids. As a group the charged amino acids are important for making proteins soluble. These residues are generally located on the surface of the protein. Arginine is well designed to bind the phosphate anion, and is often found in the active centers of proteins that bind phosphorylated substrates. As a cation, arginine, as well as lysine, plays a role in maintaining the overall charge balance of a protein. Although arginine is considered an essential amino acid (it must be obtained through the diet), this is true only during the juvenile period in humans.
- Abbreviations: Arg, R
- IUPAC name: 2-amino-5-(diaminomethylideneamino)pentanoic acid
- Molecular formula: C6H14N4O2
- Molecular weight: 174.20 g/mol
Arrhenius equation → Arheniusova jednadžba
In 1889, Svante Arrhenius explained the variation of rate constants with temperature for several elementary reactions using the relationship
where the rate constant k is the total frequency of collisions between reaction molecules A times the fraction of collisions exp(-Ea/RT) that have an energy that exceeds a threshold activation energy Ea at a temperature of T (in kelvin). R is the universal gas constant.
Citing this page:
Generalic, Eni. "Konstanta ravnoteže." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table