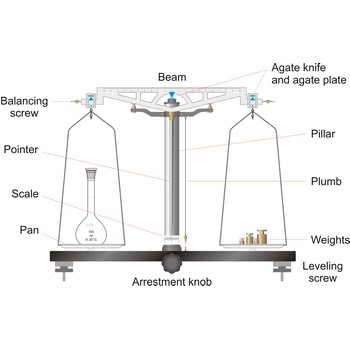
balance → vaga
Balance is an instrument to measure the mass (or weight) of a body. Balance beam type scales are the oldest type and measure weight using a fulcrum or pivot and a lever with the unknown weight placed on one end of the lever, and a counterweight applied to the other end. When the lever is balanced, the unknown weight and the counterweight are equal. The equal-arm balance consists of two identical pans hung from either end of a centrally suspended beam. The unequal-arm balance is made with one arm of the balance much longer than the other.
More modern substitution balances use the substitution principle. In this calibrated weights are removed from the single lever arm to bring the single pan suspended from it into equilibrium with a fixed counter weight. The substitution balance is more accurate than the two-pan device and enables weighing to be carried out more rapidly.
Electromagnetic force restoration balances also use a lever system but a magnetic field is used to generate the force on the opposite end of the lever and balance out the unknown mass. The current used to drive the magnetic coil is proportional to the mass of the object placed on the platform.
chemical potential → kemijski potencijal
For a mixture of substances, the chemical potential of constituent B (μB) is defined as the partial derivative of the Gibbs energy G with respect to the amount (number of moles) of B, with temperature, pressure, and amounts of all other constituents held constant.
Also called partial molar Gibbs energy. Components are in equilibrium if their chemical potentials are equal.
Gibbs free energy → Gibbsova slobodna energija
Gibbs free energy (G) is an important function in chemical thermodynamics, defined by
where H is the enthalpy, S the entropy, and T the thermodynamic temperature. Gibbs free energy is the energy liberated or absorbed in a reversible process at constant pressure and constant temperature. Sometimes called Gibbs energy and, in older literature, simply free energy.
Changes in Gibbs free energy, ΔG, are useful in indicating the conditions under which a chemical reaction will occur. If ΔG is negative the reaction will proceed spontaneously to equilibrium. In equilibrium position ΔG = 0.
half-life of a reactant → poluživot reaktanta
For a given reaction the half-life, t1/2, of a reactant is the time required for its concentration to reach a value that is the arithmetic mean of its initial and final (equilibrium) value.
Half-life is constant for first-order reactions.
Half-life is not constant for second-order reactions but rather it varies with initial concentration and k.
Hooke’s law → Hookeov zakon
Hooke’s law stating that the deformation of a body is proportional to the magnitude of the deforming force, provided that the body’s elastic limit (see elasticity) is not exceeded. If the elastic limit is not reached, the body will return to its original size once the force is removed. The law was discovered by English physicist Robert Hooke in 1676.
If a body on elastic spring is displaced from its equilibrium position (i.e. if the spring is stretched or compressed), a restitution force tries to return the body back in its equilibrium position. The magnitude of that force is proportional to the displacement of the body
Where F is the restitutional (elastic) force, x is the displacement of the body and k is the spring constant, which depends on dimensions, shape and material of the spring.
ion-product constant → ionski produkt vode
The ion-product constant. For the reaction:
the equilibrium expression would be:
Note that all pure liquid terms are omitted, hence H2O does not appear in the denominator. At 25 °C
osmotic pressure → osmotski tlak
Osmotic pressure (Π) is the excess pressure necessary to maintain osmotic equilibrium between a solution and a pure solvent separated by a membrane permeable only to the solvent. In an ideal dilute solution
where cB is the amount-of-substance concentration of the solute, R is the molar gas constant, and T the temperature.
Ostwald’s dilution law → Ostwaldov zakon razrjeđenja
Ostwald’s dilution law is a relation for the concentration dependence of the molar conductivity Λ of an electrolyte solution, viz.
where c is the solute concentration, Kc is the equilibrium constant for dissociation of the solute, and L0 is the conductivity at cΛ = 0. The law was first put forward by the German chemist Wilhelm Ostwald (1853-1932).
van’t Hoff equation → van Hoffova jednadžba
Van’t Hoff equation is the equation expressing the temperature dependence on the equilibrium constant K of a chemical reaction:
where ΔrH° is the standard enthalpy of reaction, R the molar gas constant, and T the temperature.
Citing this page:
Generalic, Eni. "Konstanta ravnoteže." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table