diffusion → difuzija
Diffusion is the spontaneous mixing of one substance with another when in contact or separated by a permeable membrane. Diffusion is a result of the random motions of their component atoms, molecules, ions, or other particles. Diffusion occurs most readily in gases, less so in liquids, and least in solids. The rate of diffusion is proportional to the concentration of the substance and increases with temperature. The theoretical principles are stated in Fick’s laws.
dissociation constant → konstanta disocijacije
Dissociation constant is a constant whose numerical value depends on the equilibrium between the undissociated and dissociated forms of a molecule. A higher value indicates greater dissociation.
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
The equilibrium constant of such a dissociation is called the acid dissociation constant or acidity constant, given by
The concentration of water [H2O] can be taken as constant.
Similarly, for a base, the equilibrium
is also a dissociation; with the base dissociation constant or basicity constant, given by
Ka (Kb) is a measure of the strength of the acid (base).
lower flammable limit → donja granica zapaljivosti
Lower flammable limit (LEL) or the lower explosive limit is the minimum concentration of vapour or gas in air above which propagation of flame does not occur on contact with a source of ignition. The mixture is said to be too lean.
molar absorption coefficient → molarni apsorpcijski koeficijent
Molar absorption coefficient (ε) is the absorption coefficient divided by amount-of-substance concentration of the absorbing material in the sample solution (ε = a/c). The SI unit is m2mol-1. Also called extinction coefficient, but usually in units of dm3cm-1mol-1.
osmosis → osmoza
Osmosis is the flow of a solvent in a system in which two solutions of different concentration are separated by a semipermeable membrane which cannot pass solute molecules. The solvent will flow from the side of lower concentration to that of higher concentration, thus tending to equalise the concentrations. The pressure that must be applied to the more concentrated side to stop the flow is called the osmotic pressure.
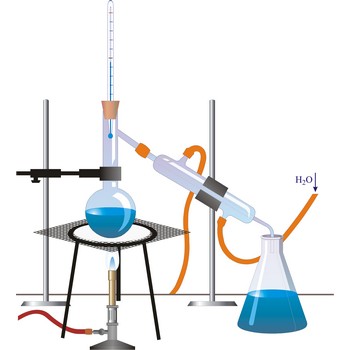
distillation → destilacija
Distillation is a process of boiling a liquid and condensing and collecting the vapour. The liquid collected is the distillate. The usual purpose of distillation is purification or separation of the components of a mixture. This is possible because the composition of the vapour is usually different from that of liquid mixture from which it is obtained. Petrol, kerosene, fuel oil, and lubricating oil are produced from petroleum by distillation.
electrode of the first kind → elektroda prvog reda
Electrode of the first kind is a simple metal electrode immersed in a solution containing its own ion (e.g., silver immersed in a silver nitrate solution). The equilibrium potential of this electrode is a function of the concentration (more correctly of activity) of the cation of the electrode metal in the solution (see Nernst’s electrode potential equation).
electrode of the second kind → elektroda drugog reda
Electrodes of the second kind are metal electrodes assembly with the equilibrium potential being a function of the concentration of an anion in the solution. Typical examples are the silver/silver-chloride electrode and the calomel electrode. The potential of the metal is controlled by the concentration of its cation in the solution, but this, in turn, is controlled by the anion concentration in the solution through the solubility product of the slightly soluble metal salt. Contrast with electrode of the first kind and electrode of the third kind.
electrode of the third kind → elektroda trećeg reda
Electrode of the third kind is a metal electrode assembly with the equilibrium potential being a function of the concentration of a cation, other than the cation of the electrode metal, in the solution. The assembly consists of a metal in contact with two slightly soluble salts (one containing the cation of the solid metal, the other the cation to be determined, with both salts having a common anion) immersed in a solution containing a salt of the second metal (e.g., zinc metal--zinc oxalate--calcium oxalate--calcium salt solution). The potential of the metal is controlled by the concentration of its cation in the solution, but this is controlled by the anion concentration in the solution through the solubility product of the slightly soluble metal salt, which, in turn is controlled by the concentration of the cation of the second slightly soluble salt. These electrodes are very sluggish and unstable due to a series of equilibria to be established to produce a stable potential.
Citing this page:
Generalic, Eni. "Koncentracija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table