Ilkovic equation → Ilkovičeva jednadžba
Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form
Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3.
The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980).
indicator electrode → indikatorska elektroda
Indicator electrode is working in one of the electrodes in some classical two-electrode cells, e.g., in a potentiometric electroanalytical setup where the potential of the measuring electrode (against a reference electrode) is a measure of the concentration (more accurately activity) of a species in the solution.
Nernst’s division law → Nernstov zakon razdjeljenja
Nernst’s division law states that a substance is divided between two solvents in a way that proportion of concentrations of that substance is at certain temperatures constant, under the condition that both solvents are in the same molecular state. Division coefficient is a proportion of substance concentration in solvents A i B at a defined temperature.
Appearance of division is used for substance extraction.
Nusselt number → Nusseltova značajka
Nusselt number (Nu) is a dimensionless quantity used in fluid mechan-ics, defined by
where h is coefficient of heat transfer, l is length, and k is thermal conductivity.
Onsager relations → Onsagerove relacije
Onsager relations are an important set of equations in the thermodynamics of irreversible processes. They express the symmetry between the transport coefficients describing reciprocal processes in systems with a linear dependence of flux (Ji) on driving forces (Xj).
In Onsager’s theory the coupling coefficients are equal, Lij = Lji. This is known as reciprocal relations. The theory was developed by the Norwegian chemist Lars Onsager (1903-1976) in 1931.
pH → pH
pH is a convenient measure of the acid-base character of a solution, usually defined by
where c(H+) is the concentration of hydrogen ions in moles per litre. The more precise definition is in terms of activity rather than concentration.
A solution of pH 0 to 7 is acid, pH of 7 is neutral, pH over 7 to 14 is alkaline.
Rayleigh number → Rayleighova značajka
Rayleigh number (Ra) is a dimensionless quantity used in fluid mechanics, defined by
where l is length, g is acceleration of gravity, α is cubic expansion coefficient, T is temperature, ρ is density, η is viscosity, and a is thermal diffusivity.
Schmidt number → Schmidtova značajka
Schmidt number (Sc) is a dimensionless quantity used in fluid mechanics, defined by
where η is viscosity, ρ is density, and D is diffusion coefficient.
solubility product constant → konstanta produkta topljivosti
Solubility product constant (Ksp) (or the solubility product) is the product of the molar concentrations of the constituent ions, each raised to the power of its stoichiometric coefficient in the equilibrium equation. For instance, if a compound AaBb is in equilibrium with its solution
the solubility product is given by
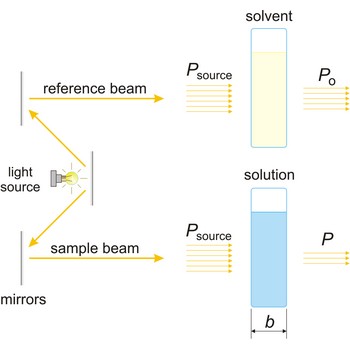
spectrophotometer → spektrofotometar
Spectrophotometer is an instrument for measuring the amount of light absorbed by a sample.
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
Citing this page:
Generalic, Eni. "Koeficijent aktiviteta." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table