detergent → deterdžent
Detergent is a substance added to water to improve its cleaning properties. Although water is a powerful solvent for many compounds, it will not dissolve grease and natural oils. Detergents are compounds that cause such nonpolar substances to go into solution in water. Soap is the original example, owing its action to the presence of ions formed from long-chain fatty acids ion (e.g. stearat ion, CH3(CH2)16COO-).
catalyst → katalizator
Catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. Catalysts that have the same phase as the reactants are homogenous catalysts (e.g. enzymes in biochemical reactions). Those that have a different phase are heterogeneous catalyst (e.g. metals or oxides used in gas reactions).
The catalyst provides an alternative pathway by which the reaction can proceed, in which the activation energy is lower. In thus increases the rate at which the reaction comes to an equilibrium, although it does not alter the position of the equilibrium.
cellulose → celuloza
Cellulose, (C6H10O5)n, is a polysaccharide that consists of a long unbranched chain of glucose units linked by (1→4)-β-glycoside bonds. Nature uses cellulose primarily as a structural material to impart strength and rigidity to plants. Leaves, grasses, and cotton are primarily cellulose. The fibrous nature of extracted cellulose has led to its use in textile industry for the production of cotton, artificial silk, etc. Cellulose also serves as raw material for the manufacture of cellulose acetate, known commercially as acetate rayon, and cellulose nitrate, known as guncotton. Gunncotton is the major ingredient in smokeless powder, the explosive propellant used in artillery shells and in ammunition for firearms.
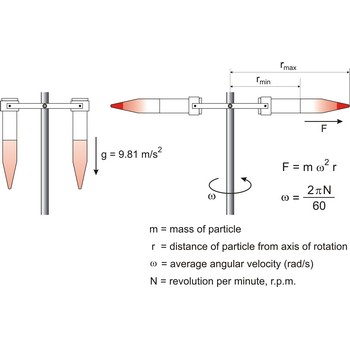
centrifuge → centrifuga
Centrifuge is a device in which solid or liquid particles of different densities are separated by rotating them in a tube in a horizontal circle. The dense particles tend to move along the length of the tube to a greater radius of rotation, displacing the lighter particles to the other end.
effervescence → pjenušanje
Effervescence is the formation of gas bubbles in a liquid by a chemical reaction. An example of effervescence is the release of carbon dioxide which bubbles as a gas from the liquid when limestone chips, which are composed of calcium carbonate, are added to dilute hydrochloric acid.
electron pair → elektronski par
Electron pair is two electrons within one orbital with opposite spins responsible for a chemical bond.
equivalent → ekvivalent
Equivalent (eq) is a unit for describing the amount of a chemical species. In contrast to the mole, the amount of a substance contained in one equivalent can vary from reaction to reaction.
cerium → cerij
Cerium was discovered by Martin Heinrich Klaproth (Germany) and by Jöns Jacob Berzelius (Sweden) in 1803 and Wilhelm von Hisinger (Germany) in 1814. Named after the asteroid Ceres this discovered two years before the element. It is malleable, ductile, iron-grey metal. Tarnishes in air; reacts easily with water. Dissolves in acids; ignites when heated. Metal ignites and burns readily. Strong reductant. Cerium is most abundant rare earth metal. Found in many minerals like monazite sand [Ce(PO4)]. Its oxides are used in the optics and glass-making industries. Its salts are used in the photography and textile industry. Used in high-intensity carbon lamps and as alloying agents in special metals.
Citing this page:
Generalic, Eni. "Kemijsko staklo." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table