methionine → metionin
Methionine is neutral amino acids with polar side chains. It is one of the two sulfur-containing amino acids. Methionine is a fairly hydrophobic amino acid and typically found buried within the interior of a protein. It can form stacking interactions with the aromatic moieties of tryptophan, phenylalanine, and tyrosine. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Met, M
- IUPAC name: 2-amino-4-methylsulfanylbutanoic acid
- Molecular formula: C5H11NO2S
- Molecular weight: 149.21 g/mol
noble gas → plemeniti plin
Noble gas refers to any element of the group of six elements in group 18 of the periodic table. They are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Unlike most elements, the noble gases are monoatomic. The atoms have stable configurations of electrons. Therefore, under normal conditions they do not form compounds with other elements.
They were generally called inert gases until about 1962 when xenon tetrafluoride, XeF4, was produced in the laboratory. This was the first report of a stable compound of a noble gas with another single element.
nucleotide → nukleotid
Nucleotides are the components that made up nucleic acids. They have three major components: the first component is a nitrogenous base, which is derivative of one of two parent compounds, pyrimidine or purine; the second is a pentose, or five carbon sugar group; the third is a unit of phosphate. Each group of three nucleotides in a gene is known as a codon. Whenever the phosphate group is not present, a nucleotide becomes a nucleoside.
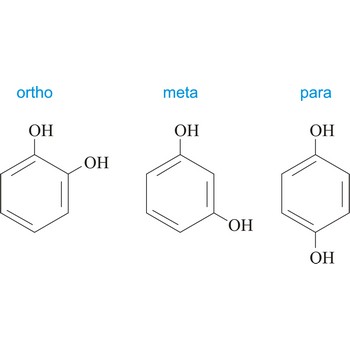
ortho position → ortho položaj
Ortho position in organic chemistry is the one in which there are two same functional groups, tied to a ring of benzene in the positions 1 and 2. The abbreviation o- is used, for example, o-Hydroquinone is 1,2-dihydroxybenzene.
osmium → osmij
Osmium was discovered by Smithson Tennant (England) in 1803. The origin of the name comes from the Greek word osme meaning smell. It is hard fine black powder or hard, lustrous, blue-white metal. Unaffected by air, water and acids. Characteristic acrid, chlorine like odour due to tetroxide compound. Osmium tetroxide highly toxic. Osmium is obtained from the same ores as platinum. Used to tip gold pen points, instrument pivots, to make electric light filaments. Used for high temperature alloys and pressure bearings. Very hard and resists corrosion better than any other.
palladium → paladij
Palladium was discovered by William Hyde Wollaston (England) in 1803. Named after the asteroid Pallas which was discovered at about the same time and from the Greek name Pallas, goddess of wisdom. It is soft, malleable, ductile, silvery-white metal. Resists corrosion; dissolves in oxidizing acids. Absorbs hydrogen. Metal dust is combustible. Palladium is obtained with platinum, nickel, copper and mercury ores. Used as a substitute for silver in dental items and jewellery. The pure metal is used as the delicate mainsprings in analog wristwatches. Also used in surgical instruments and as catalyst.
para position → para položaj
Para position in organic chemistry is the one in which there are two same functional groups tied to a ring of benzene in the position 1 and 4. The abbreviation p- is used, for example, p-Hydroquinone is 1,4-dihydroxybenzene.
periodic table of the elements → periodni sustav elemenata
Periodic table is a table of elements, written in sequence in the order of atomic number or atomic weight and arranged in horizontal rows (periods) and vertical columns (groups) to illustrate the occurrence of similarities in the properties of the elements as a periodic function of the sequence. The original form was proposed by Dmitri Mendeleev (1834-1907) in 1869, using relative atomic masses.
platinum → platina
Platinum was discovered by Antonio de Ulloa (South America) in 1735. The origin of the name comes from the Spanish word platina meaning silver. It is rare, very heavy, soft, silvery-white metal. Resists oxygen and water. Platinum is produced from deposits of native, or elemental. Used in jewellery, to make crucible and special containers and as a catalyst. Used with cobalt to produce very strong magnets. Also to make standard weights and measures. Resists corrosion and acid attacks except aqua regia.
Citing this page:
Generalic, Eni. "Karbonilna skupina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table