charge number → nabojni broj
Charge number represents ion charge of an atom or a group. It is denoted as a right superscript (Fe2+ or SO42-).
conjugated protein → konjugirani protein
Conjugated proteins are proteins which have a prostetic group as a part of their structure which is bonded with one or more amino acids of the same protein.
derivative → derivat
Derivative is a compound that is derived from some other compound and usually maintains its general structure, e.g. trichlormethane (chloroform) is a derivative of methane.
diazo compounds → diazo-spojevi
Diazo compounds are compounds having the divalent diazo group, =N+=N-, attached to a carbon atom. The term includes azo compounds, diazonium compounds, and also such compounds as diazomethane, CH2=N2.
ionisation energy → energija ionizacije
Ionisation energy is the minimum energy required to remove an electron from an isolated atom or molecule (in its vibrational ground state) in the gaseous phase.
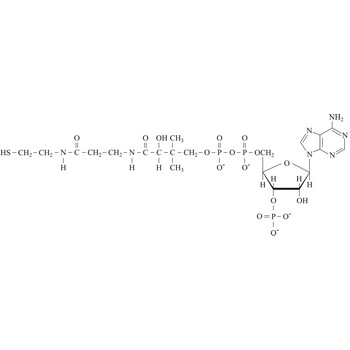
coenzyme a → koenzim a
Coenzyme A (CoA) is an essential metabolic cofactor synthesized from cysteine, pantothenate (vitamin B5), and ATP. CoA plays important roles in many metabolic pathways, including the tricarboxylic acid (TCA) cycle, and the synthesis and oxidation of fatty acids. One of the main functions of CoA is the carrying and transfer of acyl groups. Acylated derivatives (acetyl-CoA) are critical intermediates in many metabolic reactions.
coenzyme q → koenzim q
Coenzyme Q (CoQ) or ubiquinone is any of a group of related quinone-derived compounds that serve as electron carriers in the electron transport chain reactions of cellular respiration. There are some differences in the length of the isoprene unit (in bracket on left) side chain in various species. All the natural forms of CoQ are insoluble in water, but soluble in membrane lipids.
concentration → koncentracija
1. Group of four quantities characterizing the composition of a mixture with respect to the volume of the mixture (mass, amount, volume and number concentration).
2. Short form for amount (of substance) concentration (substance concentration in clinical chemistry).
cross-linking → umrežavanje
Cross-linking is an attachment of two chains of polymer molecules by bridges, composed of either an element, a group, or a compound, that join certain carbon atoms of the chains by primary chemical bonds, as indicated in the schematic diagram
Cross-linking occurs in nature in substances made up of polypeptide chains that are joined by the disulfide bonds of the cysteine residue, as in keratins or insulin. Cross-linking can be artificially effected, either adding a chemical substance (cross-linking agent), or by subjecting the polymer to high-energy radiation. Examples are: vulcanisation of rubber with sulphur, cross-linking of polystyrene with divinylbenzene, or cross-linking of polyethylene by means of high-energy radiation.
Cross-linking has the effect of changing a plastic from thermoplastic to thermosetting. Thus, it also increases strength, heat and electrical resistance, and especially resistance to solvents and other chemicals.
Citing this page:
Generalic, Eni. "Karboksilna skupina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table