extensive property → ekstenzivno svojstvo
Extensive property is a property that changes when the amount of matter in a sample changes. Examples are mass, volume, length, and charge.
fermentation → fermentacija
Fermentation is a class of biochemical reactions that break down complex organic molecules (such as carbohydrates) into simpler materials (such as ethanol, carbon dioxide, and water). Fermentation reactions are catalyzed by enzymes.
Fermi level → Fermijev nivo
Fermi level is the highest energy of occupied states in a solid at zero temperature. The Fermi level in conductors lies in the conduction band, in insulators it lies in the valence band, and in semiconductors it falls in the gap between the conduction band and the valence band. It was named after the Italian physicst Enrico Fermi (1901 - 1954).
dialysis → dijaliza
Dialysis is a method by which large molecules (such as starch or protein) and small molecules (such as glucose or amino acids) may be separated in a solution by selective diffusion through a semipermeable membrane. Through this kind of membrane dissolved particles pass and colloid dimension particles fall behind. For example, if a mixed solution of starch and glucose is placed in a closed container made of a semipermeable substance (such as cellophane), which is then immersed in a beaker of water, the smaller glucose molecules will pass trough the membrane into the water, while the starch molecules remain behind.
diamagnetism → dijamagnetizam
In diamagnetism the magnetisation is in the opposite direction to that of the applied field, i.e. susceptibility is negative. It results from changes induced in the orbits of electrons in the atoms of a substance by the applied field, the direction of the change opposing the applied flux.
dielectric constant → dielektrična konstanta
Dielectric constant or permittivity (ε) is an index of the ability of a substance to attenuate the transmission of an electrostatic force from one charged body to another. The lower the value, the greater the attenuation. The standard measurement apparatus utilises a vacuum whose dielectric constant is 1. In reference to this, various materials interposed between the charged terminal have the following value at 20 °C:
| vacuum | 1 |
| air | 1.00058 |
| glass | 3 |
| benzene | 2.3 |
| acetic acid | 6.2 |
| ammonia | 15.5 |
| ethanol | 25 |
| glycerol | 56 |
| water | 81 |
The exceptionally high value for water accounts for its unique behaviour as a solvent and in electrolytic solutions. Dielectric constant values decrease as the temperature rises.
diffusion → difuzija
Diffusion is the spontaneous mixing of one substance with another when in contact or separated by a permeable membrane. Diffusion is a result of the random motions of their component atoms, molecules, ions, or other particles. Diffusion occurs most readily in gases, less so in liquids, and least in solids. The rate of diffusion is proportional to the concentration of the substance and increases with temperature. The theoretical principles are stated in Fick’s laws.
dipole moment → dipolni moment
Electric dipole moment (μ) is a product of the positive charge and the distance between the charges. Dipole moments are often stated in debyes; The SI unit is the coulomb metre. In a diatomic molecule, such as HCl, the dipole moment is a measure of the polar nature of the bond; i.e. the extent to which the average electron charges are displaced towards one atom (in the case of HCl, the electrons are attracted towards the more electronegative chlorine atom). In a polyatomic molecule, the dipole moment is the vector sum of the dipole moments of the individual bonds. In a symmetrical molecule, such as tetrafluoromethane (CF4) there is no overall dipole moment, although the individual C-F bonds are polar.
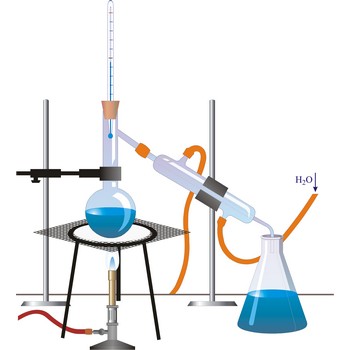
distillation → destilacija
Distillation is a process of boiling a liquid and condensing and collecting the vapour. The liquid collected is the distillate. The usual purpose of distillation is purification or separation of the components of a mixture. This is possible because the composition of the vapour is usually different from that of liquid mixture from which it is obtained. Petrol, kerosene, fuel oil, and lubricating oil are produced from petroleum by distillation.
distilled water → destilirana voda
Distilled water is water purified by distillation so as to free it from dissolved salts and other compounds. Distilled water in equilibrium with the carbon dioxide in the air has conductivity of about 0.8×10-6 S cm-1. Repeated distillation in vacuum can bring conductivity down to 0.043×10-6 S cm-1 at 18 °C. The limiting conductivity is due to self ionisation
Citing this page:
Generalic, Eni. "Kancerogene tvari." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table