polariser → polarizator
Polariser is frequently a specially made prism from crystal of Iceland Spar (form of calcium carbonate), also known as Nicol’s prism. It can also be a small plate of tourmaline.
polymorphism → polimorfija
Polymorphism is the ability of a solid substance to crystallise into more than one different crystal structure. Different polymorphs have different arrangements of atoms within the unit cell, and this can have a profound effect on the properties of the final crystallised compound. The change that takes place between crystal structures of the same chemical compound is called polymorphic transformation.
The set of unique crystal structures a given compound may form are called polymorphs. Calcium carbonate is dimorphous (two forms), crystallizing as calcite or aragonite. Titanium dioxide is trimorphous; its three forms are brookite, anatase, and rutile. The prevailing crystal structure depends on both the temperature and the external pressure.
Iron is a metal with polymorphism structure. Each structure stable in the range of temperature, for example, when iron crystallizes at 1 538 °C it is bcc (δ-iron), at 1 394 °C the structure changes to fcc (γ-iron or austenite), and at 912 °C it again becomes bcc (α-iron or ferrite).
Polymorphism of an element is called allotropy.
potassium glass → kalijevo staklo
Potassium glass is a type of glass produced from potassium silicates and calcium with potassium carbonate. It dissolves harder than regular glass and it is used in production of chemical vessels.
seawater → more
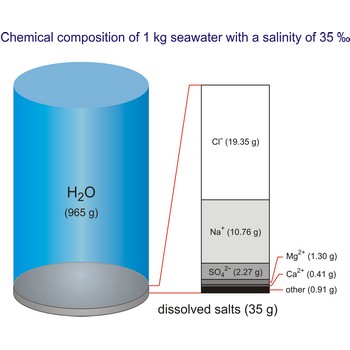
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
soft water → meka voda
Soft water is any water that does not contain large concentrations of the dissolved minerals calcium or magnesium.
water softener → omekšivač vode
Water softeners are substances which help remove constant water hardness. It reacts with calcium and magnesium salts, creating compounds that do not react with soap.
water softening → omekšavanje vode
Water softening is a process in which calcium and magnesium ions are removed from water. It is usually done by ion exchanger which exchanges removed ions with sodium ones.
Solvay’s process → Solvayev postupak
Solvay ’s process is an industrial process for producing sodium carbonate from sodium chloride and ammonia and carbon dioxide.
Carbon dioixide is produced by the thermal decomposition of limestone, CaCO3(s).
Quicklime, formed as a by-product of the thermal decomposition of limestone, is treated with water to form calcium hydroxide.
Calcium hydroxide is heated with ammonium chloride to form ammonia and calcium chloride (by product).
Carbon dioxide reacts with ammonia to form ammonium carbonate.
Ammonium carbonate further reacts with carbon dioxide to form ammonium bicarbonate.
Ammonium bicarbonate then react with sodium chloride to form sodium bicarbonate.
Dry sodium bicarbonate is heated in rotary furnace to give anhydrous sodium carbonate or soda ash.
The carbon dioxide produced is recycled back into the process.
temporary hardness → prolazna tvrdoća
Temporary Hardness is due to the bicarbonate ion, HCO3-, being present in the water. It can be removed by water reboiling, whereby white solid emerges calcium carbonate that is limescale.
ununquadium → ununkvadij
The discovery of ununquadium was reported informally in January 1999 following experiments towards the end of December 1998 involving scientists at Dubna (Joint Institute for Nuclear Research) in Russia and the Lawrence Livermore National Laboratory, USA. The new element has not yet been officially named, but it is known as ununquadium, according to the system designated by the IUPAC for naming new elements. It is synthetic radioactive metal. Only few atoms of element 114 (289114) has ever been made (through a nuclear reaction involving fusing a calcium atom with a plutonium atom) isolation of an observable quantity has never been achieved.
Citing this page:
Generalic, Eni. "Kalcij." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table