Results 1–9 of 9 for kadmij
cadmium → kadmij
Cadmium was discovered by Friedrich Strohmeyer (Germany) in 1817. The origin of the name comes from the Latin word cadmia meaning calamine (zinc carbonate, ZnCO3), or from the Greek word kadmeia with the same meaning. It is soft, malleable, blue-white metal. Tarnishes in air, soluble in acids, insoluble in alkalis. Boiling cadmium gives off a weird, yellow-colored vapour that is poisonous. Cadmium can cause a variety of health problems, including kidney failure and high blood pressure. Cadmium is obtained as a by product of zinc refining. The mayor use of cadmium is in electroplating of steel to protect it from corrosion. Also used to make nickel-cadmium batteries. The ability of cadmium to adsorb neutrons has made it of great importance in the design of nuclear reactors. Its compounds are found in paint pigments and a wide variety of intense colours.
cadmium coating → kadmiranje
Cadmium coating is a galvanic coating of metallic objects with a cadmium layer for corrosion resistance.
accumulator → akumulator
Accumulator (secondary cell, storage battery) is a type of voltaic cell or battery that can be recharged by passing current through it from an external D.C. supply. The charging current reverses the chemical reactions in the cell. The common types are the lead-acid accumulator and the nickel-cadmium cell.
bismuth → bizmut
Bismuth was discovered by Claude Geoffroy (France) in 1753. The origin of the name comes from the German words Weisse Masse meaning white mass; now spelled wismut and bisemutum. It is hard, brittle, steel-grey metal with a pink tint. Stable in oxygen and water. Dissolves in concentrated nitric acid. Bismuth can be found free in nature and in minerals like bismuthine (Bi2S3) and in bismuth ochre (Bi2O3) Main use is in pharmaceuticals and low melting point alloys used as fuses.
control rod → kontrolna šipka
Control rods are rods in a nuclear reactor composed of substances that absorb neutrons (cadmium, hafnium, boron, etc.). These rods regulate the power level of the reactor.
dysprosium → disprozij
Dysprosium was discovered by Paul Emile Lecoq de Boisbaudran (France) in 1886. The origin of the name comes from the Greek word dysprositos meaning hard to obtain. It is soft, lustrous, silvery metal. Reacts with oxygen. Reacts rapidly with water; dissolves in acids. Metal ignites and burns readily. Reductant. Dysprosium usually found with erbium, holmium and other rare earths in some minerals such as monazite sand. Dysprosium uses are limited to the experimental and esoteric. Some isotopes of dysprosium are effective absorbers of thermal neutrons and are being considered for use in the control rods in nuclear reactors.
mercury → živa
Mercury has been known since ancient times. The origin of the name comes from the Latin word hydrargyrum meaning liquid silver. It is heavy, silver-white metal, liquid at ordinary temperatures. Stable in air and water. Unreactive with alkalis and most acids. Gives off poisonous vapour. Chronic cumulative effects. Mercury only rarely occurs free in nature. The chief ore is cinnabar or mercury sulfide (HgS). Used in thermometers, barometers and batteries. Also used in electrical switches and mercury-vapour lighting products.
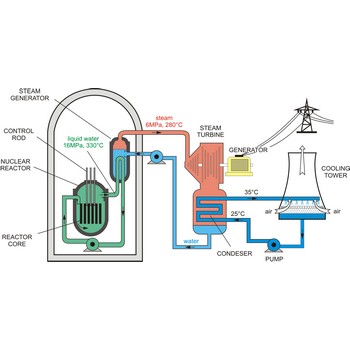
nuclear reactor → nuklearni reaktor
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
Citing this page:
Generalic, Eni. "Kadmij." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table