calorimetry → kalorimetrija
Calorimetry is a measurement of the amount of heat evolved or absorbed in a chemical reaction, change of state, or formation of a solution, or any other event that includes heat transfer.
chemical raw material → kemijske sirovine
Chemical raw material are petroleum fractions used for obtaining organic chemicals, those are mostly refined gas and petroleum or fraction parts of petrol.
chemical reaction → kemijska reakcija
Chemical reaction is a change of chemical properties of substances which react with each other. By means of a chemical reaction new substances are created by bond breaking between atoms and molecules of reactants and their reuniting in a new way, thereby creating products. Chemical reactions can be shown by chemical equations.
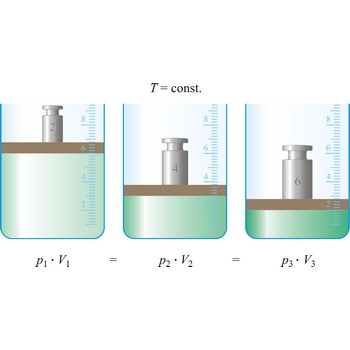
Boyle’s law → Boyleov zakon
Boyle’s law (sometimes referred to as the Boyle-Mariott’s law) is the empirical law, exact only for an ideal gas, which states that the volume of a gas is inversely proportional to its pressure at constant temperature.
Bragg angle → Braggov kut
Bragg angle (Θ) is the angle between an incident X-ray beam and a set of crystal planes for which the secondary radiation displays maximum intensity as a result of constructive interference. British physicist Sir William Henry Bragg and his son Sir William Lawrence Bragg developed a simple relation for scattering angles, now call Bragg’s law.
which relates the angle θ between a crystal plane and the diffracted X-ray beam, the wavelength λ of the x-rays, the crystal plane spacing d, and the diffraction order n (any integer).
The diffraction experiment as presently considered is intended to provide quantitative information on the lattice constant and shape characteristics of the unit cell.
Bunsen burner → Bunsenov plamenik
Bunsen burner is a standard source of heat in the laboratory. German chemist Roberts Bunsen (1811-1899) improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. The Bunsen burner has a vertical metal tube through which a fine jet of fuel gas is directed. Air is drawn in through airholes near the base of the tube and the mixture is ignited and burns at the tube’s upper opening. The flow of this air is controlled by an adjustable collar on the side of the metal tube. When the whole is closed a yellow safety flame is displayed. Where as when the whole is open it displays a power dull blue flame with a faint blue outer flame with a vibrant blue core used u for combustion and hearting. The flame can reach temperatures of 1 500 °C.
chemical symbols → kemijski simboli
Chemical symbols are a derived way of showing elements in a formula or equation. Each symbol represents one atom and it usually consists of the first two letters of the Greek or Latin name of the element.
chemisorption → kemisorpcija
Chemisorption is a binding of a liquid or gas on the surface or in the interior of a solid by chemical bonds or forces.
chlorination → kloriranje
1. Chlorination is an addition or substitution of chlorine in organic compounds.
2. Chlorination is a sterilisation of drinking and swimming pool water or oxidation of undesirable impurities, using chlorine or its compounds.
Carnot cycle → Carnotov kružni proces
Carnot cycle is the most efficient cycle of operations for a reversible heat engine. Published in 1824 by French physicist Nicolas Léonard Sadi Carnot (1796-1832), it consists of four operations on the working substance in the engine:
1-2: Isothermal expansion at thermodynamic temperature T1 with heat QH taken in.
2-3: Adiabatic expansion with a fall of temperature to T2.
3-4: Isothermal compression at temperature T2 with heat QC given out.
4-1: Adiabatic compression at temperature back to T1.
According to the Carnot principle, the efficiency of any reversible heat engine depends only on the temperature range through which it works, rather than the properties of the working substances.
Citing this page:
Generalic, Eni. "Jednadžba stanja idealnog plina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table