Onsager relations → Onsagerove relacije
Onsager relations are an important set of equations in the thermodynamics of irreversible processes. They express the symmetry between the transport coefficients describing reciprocal processes in systems with a linear dependence of flux (Ji) on driving forces (Xj).
In Onsager’s theory the coupling coefficients are equal, Lij = Lji. This is known as reciprocal relations. The theory was developed by the Norwegian chemist Lars Onsager (1903-1976) in 1931.
Ostwald’s dilution law → Ostwaldov zakon razrjeđenja
Ostwald’s dilution law is a relation for the concentration dependence of the molar conductivity Λ of an electrolyte solution, viz.
where c is the solute concentration, Kc is the equilibrium constant for dissociation of the solute, and L0 is the conductivity at cΛ = 0. The law was first put forward by the German chemist Wilhelm Ostwald (1853-1932).
Newton’s gravitational law → Newtonov zakon gravitacije
Every object in the universe attracts every other object with a force (gravitational force FG) directed along the line through centres of the two objects that is proportional to the product of their masses and inversely proportional to the square of the distance between them.
m1 and m2 are masses of the two objects and r is the distance between them. G is universal constant of gravitation, which equals 6.67•10-26 N m2 kg-2. Strictly speaking, this law applies only to objects that can be considered pointlike object. Otherwise, the force has to be found by integrating the forces between various mass elements.
It is more properly to express Newton’s gravitational law by vector equation:
in which r1 and r2 are position vectors of masses m1 and m2.
Gravitational forces act on distance. Newton’s gravitational law is derived from Kepler’s law for planetary motion, using a physical assumption considering Sun as the centre and the source of gravitational force.
Additionally, every object moves in the direction of the force acting on it, with acceleration that is inversely proportional to the mass of object. For bodies on the surface of Earth, the distance r in gravitational law formula is practically equal to the Earth radius, RE. If the mass of the body on Earth surface is m and the mass of earth is ME, the gravitational force acting on that body can be expressed as:
where g is gravitational acceleration which is, although dependent on geographical latitude, usually considered as constant equal to 9.81 m s-2.
oxidation number → oksidacijski broj
Atoms can give one or more electrons for bond forming. The valence of any atom, which comes from stechiometrical relation of interbonded atoms, is called an oxidation number or an oxidation degree. Oxidation number of atoms in elementary state is zero. An atom of greater electronegativity has a negative, and an atom of lesser electronegativity has a positive oxidation number.
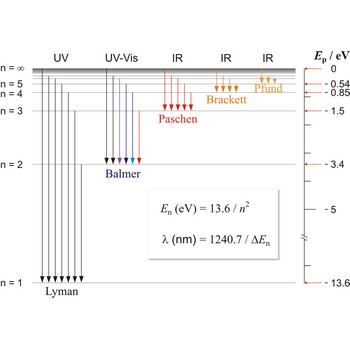
Paschen series → Paschenova serija
Paschen series are the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the state with principal quantum number n = 3 and successive higher states.
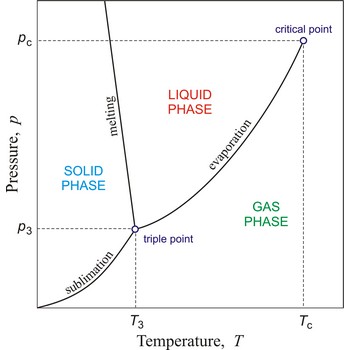
phase diagram → fazni dijagram
Phase diagram is a graphic representation of the equilibrium relationships between phases (such as vapour-liquid, liquid-solid) of a chemical compound, mixture of compounds, or solution.
The figure shows a typical phase diagram of an element or a simple compound. The stability of solid, liquid and gas phases depends on the temperature and the pressure. The three phases are in equilibrium at the triple point. The gas and liquid phases are separated by a phase transition only below the temperature of the critical point.
phosphorescence → fosforescencija
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the exciting energy has been removed. Unlike fluorescence, in which the absorbed energy is spontaneously emitted about 10-8 second after excitation, phosphorescence requires additional excitation to produce radiation and may last from about mili second to days or years, depending on the circumstances.
photoelectric effect → fotoelektrični efekt
Photoelectric effect is the complete absorption of a photon by a solid with the emission of an electron. The energy of a photon (hν) is
plasma → plazma
Plasma is a highly ionised gas in which the charge of the electrons is balanced by the charge of the positive ions, so that the system as a whole is electrically neutral. Plasmas are created by exposing gases at low pressure to an electric or electromagnetic field. In semiconductor processing, plasmas are used for etching and thin film deposition (the excited state of the gas makes it very reactive). In everyday life plasmas are used to give light in fluorescent light bulbs, neon lamps, and blue insect traps.
potential energy → potencijalna energija
Potential energy (Ep) is the energy stored in a body or system as a consequence of its position, shape, or state (this includes gravitation energy, electrical energy, nuclear energy, and chemical energy). Gravitational potential energy is the energy associated with the state of separation between bodies that attracts each other via gravitational force. Elastic potential energy is the energy associated with the state of compression or extension of an elastic object. Thermal energy is associated with the random motions of atoms and molecules in a body.
Citing this page:
Generalic, Eni. "Jednadžba stanja." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table