fume hood → digestor
Fume hood is a type of local exhaust ventilation system (engineering control). A typical fume hood is cabinet with a moveable front sash (window) made out of safety glass. Air is drawn into the hood under and through the opened sash and is exhausted through openings in the rear and top of the cabinet to a remote point such as an exhaust stack on the roof of the building. A properly used and properly functioning fume hood exhausts hazardous gases, dusts, mists, and vapors from a confined location and helps protect workers from inhalation exposure.
Gibbs free energy → Gibbsova slobodna energija
Gibbs free energy (G) is an important function in chemical thermodynamics, defined by
where H is the enthalpy, S the entropy, and T the thermodynamic temperature. Gibbs free energy is the energy liberated or absorbed in a reversible process at constant pressure and constant temperature. Sometimes called Gibbs energy and, in older literature, simply free energy.
Changes in Gibbs free energy, ΔG, are useful in indicating the conditions under which a chemical reaction will occur. If ΔG is negative the reaction will proceed spontaneously to equilibrium. In equilibrium position ΔG = 0.
glass electrode → staklena elektroda
Glass electrode is a hydrogen-ion responsive electrode usually consisting of a bulb, or other suitable form, of special glass attached to a stem of high resistance glass complete with internal reference electrode and internal filling solution system. Glass electrode is also available for the measurement of sodium ions.
The glass electrode, which consists of a thin wall glass bulb, has an extremely high electrical resistance. The membrane of a typical glass electrode (with a thickness of 0.03 mm to 0.1 mm) has an electrical resistance of 30 MΩ to 600 MΩ. The surface of a glass membrane must be hydrated before it will function as a pH electrode. When a glass surface is immersed in an aqueous solution then a thin solvated layer (gel layer) is formed on the glass surface in which the glass structure is softer. This applies to both the outside and inside of the glass membrane.
The simplest explanation for the working of the thin glass electrode is that the glass acts as a weak acid (Glass-H).
The hydrogen ion activity of the internal solution is held constant. When a solution of different pH from the inside comes in contact with the outside of the glass membrane, the glass is either deprotonated or protonated relative to the inside of the glass. The difference in pH between solutions inside and outside the thin glass membrane creates electromotive force in proportion to this difference in pH.
glutamic acid → glutaminska kiselina
Glutamic acid is an electrically charged amino acids. It is one of the two amino acids that contain a carboxylic acid group in its side chains. These acids play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Glutamic acid is commonly referred to as glutamate, because its carboxylic acid side chain will be deprotonated and thus negatively charged in its anionic form at physiological pH. Glutamic acid is referred to as a non-essential amino acid because a healthy human can synthesize all the glutamic acid needed for normal body function from other amino acids.
- Abbreviations: Glu, E
- IUPAC name: 2-aminopentanedioic acid
- Molecular formula: C5H9NO4
- Molecular weight: 147.13 g/mol
glycogen → glikogen
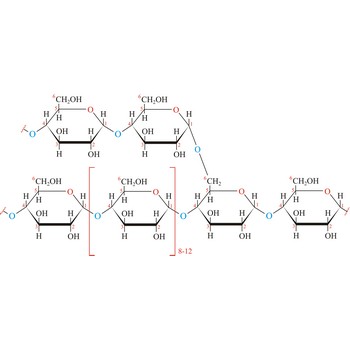
Glycogen (animal starch) is a polysaccharide that serves the same energy storage function in animals that starch serves in plants. Dietary carbohydrates not needed for immediate energy are converted by the body to glycogen for long term storage (principally in muscle and liver cells). Like amylopectin found in starch, glycogen is a polymer of α(1→4)-linked subunits of glucose, with α(1→6)-linked branches. Glycogen molecules are larger than those of amylopectin (up to 100 000 glucose units) and contain even more branches. Branch points occur about every 10 residues in glycogen and about every 25 residues in amylopectin. The branching also creates lots of ends for enzyme attack and provides for rapid release of glucose when it is needed.
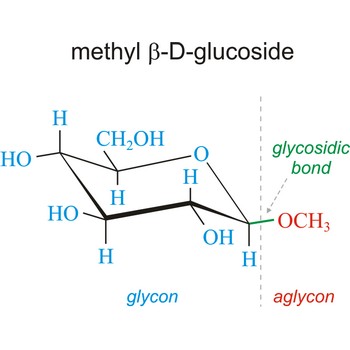
glycosidic bond → glikozidna veza
Glycosidic bond ia a bond between the glycosyl group, the structure obtained by removing the hydroxy group from the hemiacetal function of a monosaccharide, and the -OR group (which itself may be derived from a saccharide and chalcogen replacements thereof (RS–, RSe–). The terms N-glycosides and C-glycosides are misnomers and should not be used. The glycosidic bond can be α or β in orientation, depending on whether the anomeric hydroxyl group was α or β before the glycosidic bond was formed and on the specificity of the enzymatic reaction catalyzing their formation. Once the glycosidic bond is formed, the anomeric configuration of the ring is locked as either α or β. Specific glycosidic bonds therefore may be designated α(1→4), β(1→4), α(1→6), and so on. Cellulose is formed of glucose molecules linked by β(1→4)-glycosidic bonds, whereas starch is composed of α(1→4)-glycosidic bonds.
hybridization → hibridizacija
Hybridization is an internal linear combination of atomic orbitals, in which the wave functions of the atomic orbitals are added together to generate new hybrid wave functions. The new orbitals which are formed are hybrids of the originals and have properties (shape, size and energy) that are somewhere in between.
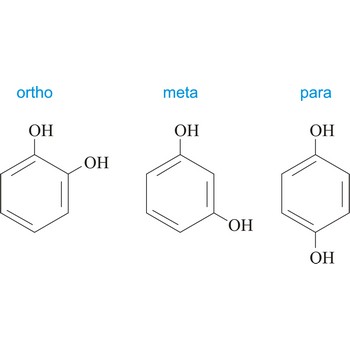
meta position → meta položaj
Meta position in organic chemistry is the one in which there are two same functional groups tied to a ring of benzene in position 1 and 3. The abbreviation m- is used, for example, m-Hydroquinone is 1,3-dihydroxybenzene.
ortho position → ortho položaj
Ortho position in organic chemistry is the one in which there are two same functional groups, tied to a ring of benzene in the positions 1 and 2. The abbreviation o- is used, for example, o-Hydroquinone is 1,2-dihydroxybenzene.
para position → para položaj
Para position in organic chemistry is the one in which there are two same functional groups tied to a ring of benzene in the position 1 and 4. The abbreviation p- is used, for example, p-Hydroquinone is 1,4-dihydroxybenzene.
Citing this page:
Generalic, Eni. "Javascript hashing function." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table