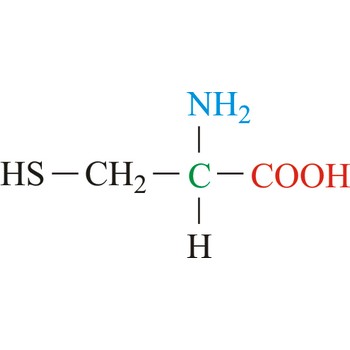
cysteine → cistein
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
glycosyl group → glikozidna skupina
The structure obtained by removing the hydroxy group from the hemiacetal function of a monosaccharide.
Helmholz free energy → Helmholzova slobodna energija
Helmholz free energy (A) is a thermodynamic function defined by A = U - TS, where U is the internal energy, S the entropy, and T the thermodynamic temperature. For a reversible isothermal process ΔA represents the useful work available.
electrical double layer → električni dvosloj
Electrical double layer is the structure of charge accumulation and charge separation that always occurs at the interface when an electrode is immersed into an electrolyte solution. The excess charge on the electrode surface is compensated by an accumulation of excess ions of the opposite charge in the solution. The amount of charge is a function of the electrode potential. This structure behaves essentially as a capacitor. There are several theoretical models that describe the structure of the double layer. The three most commonly used ones are the Helmholtz model, the Gouy-Chapman model, and the Gouy-Chapman-Stern model.
electrode of the first kind → elektroda prvog reda
Electrode of the first kind is a simple metal electrode immersed in a solution containing its own ion (e.g., silver immersed in a silver nitrate solution). The equilibrium potential of this electrode is a function of the concentration (more correctly of activity) of the cation of the electrode metal in the solution (see Nernst’s electrode potential equation).
electrode of the second kind → elektroda drugog reda
Electrodes of the second kind are metal electrodes assembly with the equilibrium potential being a function of the concentration of an anion in the solution. Typical examples are the silver/silver-chloride electrode and the calomel electrode. The potential of the metal is controlled by the concentration of its cation in the solution, but this, in turn, is controlled by the anion concentration in the solution through the solubility product of the slightly soluble metal salt. Contrast with electrode of the first kind and electrode of the third kind.
electrode of the third kind → elektroda trećeg reda
Electrode of the third kind is a metal electrode assembly with the equilibrium potential being a function of the concentration of a cation, other than the cation of the electrode metal, in the solution. The assembly consists of a metal in contact with two slightly soluble salts (one containing the cation of the solid metal, the other the cation to be determined, with both salts having a common anion) immersed in a solution containing a salt of the second metal (e.g., zinc metal--zinc oxalate--calcium oxalate--calcium salt solution). The potential of the metal is controlled by the concentration of its cation in the solution, but this is controlled by the anion concentration in the solution through the solubility product of the slightly soluble metal salt, which, in turn is controlled by the concentration of the cation of the second slightly soluble salt. These electrodes are very sluggish and unstable due to a series of equilibria to be established to produce a stable potential.
wavefunction → valna funkcija
Wavefunction (Ψ) is a mathematical function that gives the amplitude of a wave as a function of position (and sometimes as a function of time and/or electron spin). Wavefunctions are used in chemistry to represent the behaviour of electrons bound in atoms or molecules.
enzyme → enzim
Enzyme is a protein that acts as a catalyst in biochemical reactions. Each enzyme is specific to a particular reaction or a group of similar reactions. Many require the association of certain nonprotein cofactors in order to function. The molecule undergoing a reaction (the substrate) binds to a specific active site on the enzyme molecule to form a short-lived intermediate: this greatly increases (by a factor of up to 1020) the rate at which the reaction proceeds to form the product.
fugacity → fugacitet
Fugacity (f) is a thermodynamic function used in place of partial pressure in reactions involving real gases and mixtures. For a component of a mixture, it is defined by
where μ is the chemical potential.
The fugacity of a gas is equal to the pressure if the gas is ideal. The fugacity of a liquid or solid is the fugacity of the vapour with which it is in equilibrium. The ratio of the fugacity to the fugacity in some standard state is the activity.
Citing this page:
Generalic, Eni. "Javascript hashing function." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table