antimony → antimon
Antimony has been known since ancient times. The origin of the name comes from the Latin word stibium meaning mineral stibnite. It is hard, brittle, silvery-white semimetal. Stable in dry air. Toxic by ingestion or inhalation. Antimony is found in stibnite (Sb2S3) and in valentinite (Sb2O3). It is alloyed with other metals to increase their hardness. Also in the manufacture of a few special types of semiconductor devices. Also in plastics and chemicals. A few kinds of over-the-counter cold and flu remedies use antimony compounds.
Avogadro constant → Avogadrova konstanta
Avogadro constant (NA or L) is the number of elementary entities in one mole of a substance.
It has the value (6.022 045±0.000 031)×1023 mol-1.
base-centered monoclinic lattice → bazno centrirana monoklinska rešetka
Base-centered or side-centered or end-centered monoclinic lattice (monoclinic-C), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of two parallel sides of the unit cell. It has unit cell vectors a≠b≠c, and interaxial angles α=γ=90°≠β.
Bravais lattice → Bravaisova rešetka
Bravais lattice is a set of points constructed by translating a single point in discrete steps by a set of basis vectors. The French crystallographer Auguste Bravais (1811-1863) established that in three-dimensional space only fourteen different lattices may be constructed. All crystalline materials recognised till now fit in one of these arrangements. The fourteen three-dimensional lattices, classified by crystal system, are shown to the bottom.
|
Crystal system
|
Bravais lattices
|
|||
|
cubic a=b=c α=β=γ=90° |
 |
 |
 |
|
|
|
simple cubic
|
body-centered cubic
|
face-centered cubic
|
|
|
tetragonal a=b≠c α=β=γ=90° |
 |
 |
||
|
|
simple tetragonal
|
body-centered tetragonal
|
||
|
orthorhombic a≠b≠c α=β=γ=90° |
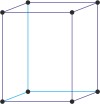 |
 |
 |
 |
|
|
simple orthorhombic
|
base-centered orthorhombic
|
body-centered orthorhombic
|
face-centered orthorhombic
|
|
monoclinic a≠b≠c α=γ=90°≠β |
 |
 |
||
|
|
simple monoclinic
|
base-centered monoclinic
|
||
|
hexagonal a=b≠c α=β=90° γ=120° |
 |
|||
|
|
hexagonal
|
|||
|
rhombohedral a=b=c α=β=γ≠90° |
 |
|||
|
|
rhombohedral
|
|||
|
triclinic a≠b≠c α≠β≠γ≠90° |
 |
|||
|
triclinic
|
||||
calcination → kalciniranje
Calcination is a process of driving out crystal water from crystal salts by heating.
base-centered orthorhombic lattice → bazno centrirana ortorompska rešetka
Base-centered or side-centered or end-centered monoclinic lattice (orthorhombic-C), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of two parallel sides of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
body-centered cubic lattice → prostorno centrirana kubična rešetka
Body-centered cubic lattice (bcc or cubic-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a = b = c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the bcc structures the spheres fill 68 % of the volume. The number of atoms in a unit cell is two (8 × 1/8 + 1 = 2). There are 23 metals that have the bcc lattice.
body-centered orthorhombic lattice → prostorno centrirana ortorompska rešetka
Body-centered orthorhombic lattice (orthorhombic-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
Citing this page:
Generalic, Eni. "Izotropni kristal." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table




