fractional crystallisation → frakcijska kristalizacija
Fractional crystallisation is a method of separating a mixture of soluble solids by dissolving them in a suitable hot solvent and then lowering the temperature slowly. The least soluble component will crystallise out first, leaving the other components in the solution. By controlling the temperature, it is sometimes possible to remove each component in turn.
heat of crystallization → toplina kristalizacije
Heat of crystallization or enthalpy of crystallization is the heat evolved or absorbed when one mole of given substance crystallises from a saturated solution of the same substance.
lattice → kristalna rešetka
Crystal lattice is a three-dimensional array of points that embodies the pattern of repetition in a crystalline solid. Don’t mix up atoms with lattice points: lattice points are infinitesimal points in space - atoms are physical objects.
lattice constants → konstante kristalne rešetke
Lattice constants are parameters specifying the dimensions of a unit cell in a crystal lattice, specifically the lengths of the cell edges and the angles between them.
lattice energy → energija kristalne rešetke
Lattice energy is the energy per ion pair required to separate completely the ions in a crystal lattice at a temperature of absolute zero.
crystal system → kristalni sustav
Crystal system is a method of classifying crystalline substances on the basis of their unit cell. There are seven unique crystal systems. The simplest and most symmetric, the cubic (or isometric) system, has the symmetry of a cube. The other six systems, in order of decreasing symmetry, are hexagonal, tetragonal, rhombohedral (also known as trigonal), orthorhombic, monoclinic and triclinic.
|
Crystal system
|
Unit-cell
|
Conditions on unit-cell edges and angles |
|
cubic |
 |
a=b=c α=β=γ=90° |
|
hexagonal |
 |
a≠c α=γ=90° β=120° |
|
tetragonal |
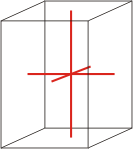 |
a=b≠c α=β=γ=90° |
|
rhombohedral |
 |
a=b=c α=β=γ≠90° |
|
orthorhombic |
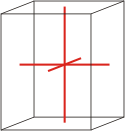 |
a≠b≠c α=β=γ=90° |
|
monoclinic |
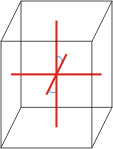 |
a≠b≠c α=γ=90°≠β |
|
triclinic |
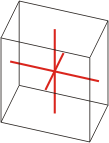 |
a≠b≠c α≠β≠γ≠90° |
cubic crystal system → kubični kristalni sustav
Cubic crystal system is also known as the isometric system. The Isometric crystal system characterizes itself by its three equivalent crystallographic axes perpendicular to each other.
a = b = c
α = β = γ = 90°
X-ray crystallography → rendgenska kristalografija
X-ray crystallography is a determination of a three dimensional arrangements of atoms in a crystal by analysis of X-ray diffraction patterns.
hexagonal crystal system → heksagonski kristalni sustav
Hexagonal crystal system is based on four crystallographic axes. The system of crystallographic axes of the hexagonal crystal system consists of three equivalent horizontal (equatorial) axes of which the positive ends make an angle of 120°. These axes are sometimes denoted as a, b and d axes. The fourth axis is (c) is perpendicular to and shorter or longer than the other three.
monoclinic crystal system → monoklinski kristalni sustav
Minerals of the monoclinic crystal system are referred to three unequal axes. Two of these axes (a and c) are inclined toward each other at an oblique angle; these are usually depicted vertically. The third axis (b) is perpendicular to the other two and is called the ortho axis. The two vertical axes therefore do not intersect one another at right angles, although both are perpendicular to the horizontal axis.
a ≠ b ≠ c
α = γ = 90° ≠ β
Citing this page:
Generalic, Eni. "Izotropni kristal." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table



